Session Information
Date: Tuesday, October 28, 2025
Title: (1855–1876) Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is an autoimmune disease characterized by type I interferon (IFN-I) production and mitochondrial dysfunction. Emerging evidence suggests that activation of the stimulator of interferon genes (STING) pathway in SSc is triggered by cytosolic mitochondrial DNA (mtDNA) released during mitochondrial stress. This link is further supported by the similarities between SSc and SAVI (STING-associated vasculopathy with onset in infancy) syndrome, a condition caused by a gain-of-function mutation in TMEM173 (which encodes STING) that phenocopies many key features of SSc. Nevertheless, the role of mitochondrial damage in activating cGAS-STING and driving IFN-I production in SSc remains poorly understood. This study aims to investigate whether mitochondrial dysfunction activates the cGAS-STING pathway and drives IFN-I production and fibrosis in SSc.
Methods: Transcriptomic analysis was performed on RNA-seq data (GSE231692, FaSScinate trial) to assess mitochondrial, cGAS-STING, and interferon-related gene expression. Skin biopsies from SSc patients and healthy controls (n=10 per group) were analyzed by RT-PCR and immunohistochemistry (IHC) for cGAS, STING, IRF3, and IFN-I. Mitochondrial dysfunction was evaluated through TFAM and MTND1 expression, Seahorse metabolic profiling, and ATP quantification in fibroblasts. To examine STING activation, fibroblasts were treated with the mitochondrial uncoupler FCCP, followed by confocal microscopy and IFNB1 quantification. In vivo relevance was tested in bleomycin-induced fibrosis (BIF) mice, with or without treatment with the selective STING inhibitor H-151.
Results: SSc skin exhibited upregulation of STING1, cGAS, TBK1, IFI16, and STAT1 compared to controls, with overexpression of STING, cGAS, and IFNB1 confirmed by RT-PCR (p < 0.01) and IHC. Mitochondrial dysfunction was evident in SSc fibroblasts, with significantly reduced TFAM expression (p < 0.01), impaired oxidative phosphorylation, decreased ATP levels, and increased glycolytic activity. FCCP-induced mitochondrial stress promoted STING nuclear translocation and IFNB1 induction, more pronounced in SSc than in control fibroblasts supporting the idea of a direct link between mitochondrial dysfunction, STING activation, and IFNβ production. Bleomycin-treated mouse models mirrored these findings, with STING-related genes (Irf3, Tmem173) being upregulated, and antioxidant defenses (Sod1, Prdx3) diminished. Treatment with H-151 significantly reduced dermal thickness and collagen deposition, underscoring the role of STING in fibrosis and the possible therapeutic potential of targeting the STING pathway in SSc.
Conclusion: Mitochondrial dysfunction in SSc promotes activation of the cGAS-STING pathway and IFN-I production, contributing to fibrotic remodeling. Pharmacologic inhibition of STING attenuates skin fibrosis in vivo, supporting STING as a novel therapeutic target in systemic sclerosis.
 A. Volcano plot illustrating upregulated and downregulated gene in RNAseq of SSc skin vs HCs. B. Heatmap of the expression of mitochondrial, cGAS-STING and Interferon-associated genes across SSc vs HCs skin.
A. Volcano plot illustrating upregulated and downregulated gene in RNAseq of SSc skin vs HCs. B. Heatmap of the expression of mitochondrial, cGAS-STING and Interferon-associated genes across SSc vs HCs skin.
C. Representative immunohistochemistry image of skin tissue showing overexpression of Sting in SSc compared to HC. D. On the left, mitostress analysis showing increased mean basal respiration and reduced spare respiratory capacity (SRC) of five control and five paired lesional SSc fibroblast cultures; on the right, rtPCR result showing TFAM downregulation in SSc skin compared to HC. E. Representative immunofluorescence image showing nuclear Sting translocation at 1 and 5 umol FCCP concentration compared to HC. F. The graph shows significant upregulation of Interferon Beta 1 gene in scleroderma fibroblasts 4 and 8 hours after FCCP stimulation, linking mitochondrial damage to interferon induction.”Error bars indicate standard deviation. Asterisks denote statistically significant differences (*P < 0.05; **P < 0.005).
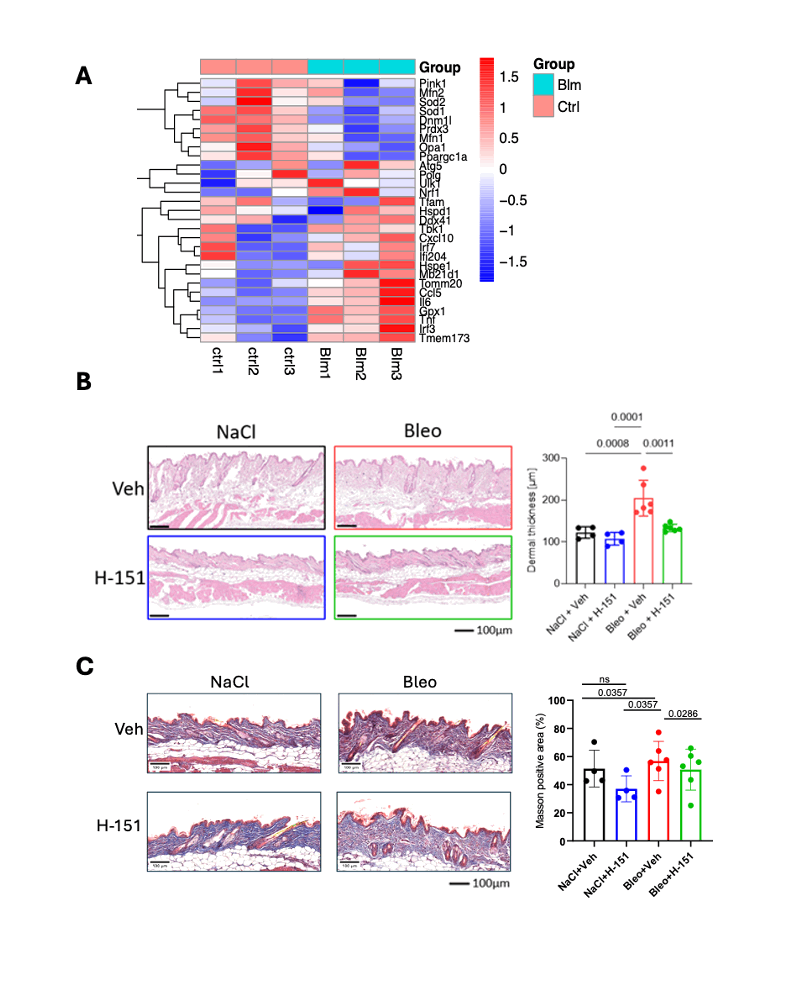
.gif) A. Heatmap from a public-available dataset showing downregulation of different mitochondrial-related genes and upregulation of Sting and Interferon type I related genes in bleomycin-treated mice compared to controls.
A. Heatmap from a public-available dataset showing downregulation of different mitochondrial-related genes and upregulation of Sting and Interferon type I related genes in bleomycin-treated mice compared to controls.
B. Quantification in H&E staining of dermal thickness in bleomycin-treated mice compared to control mice as indicated (n = 6 per group) showing dramatic improvement of dermal thickness following Sting inhibitor H-151 administration.
C. Masson Trichrome staining showing increased collagen (blue) in both control and Bleomycin-treated mice with a reduction in collagen following administration of H151.
Error bars indicate standard deviation. Asterisks denote statistically significant differences (*P < 0.05; **P < 0.005).
To cite this abstract in AMA style:
Forte G, Liakouli V, Salzillo A, Angeli M, Mauro D, Ciancio A, De Marino B, Panarese I, Faenza M, giacomelli r, Ramming A, Ciccia F. Mitochondrial Dysfunction Drives cGAS-STING–Mediated Type I Interferon Production and Fibrosis in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/mitochondrial-dysfunction-drives-cgas-sting-mediated-type-i-interferon-production-and-fibrosis-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mitochondrial-dysfunction-drives-cgas-sting-mediated-type-i-interferon-production-and-fibrosis-in-systemic-sclerosis/
