Session Information
Date: Sunday, November 12, 2023
Title: (0691–0721) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Platelets have been suggested to be involved in polymyalgia rheumatica (PMR) pathogenesis with elevated platelet activation observed in early stages of the disease. Upon platelet activation, mitochondria are expelled into the extracellular space. Extracellular mitochondria, due to their prokaryotic origin, are immunogenic and promote inflammation, as well as serve as a source of autoantigens leading to formation of anti-mitochondrial antibodies (AMAs). The purpose of this study was to explore whether patients with PMR have elevated plasma levels of thrombospondin-1 (TSP-1)—an alpha granule protein of human platelets, presence of the extracellular mitochondrial component N-formyl methionine peptide (fMET) in plasma, and autoantibodies directed towards specific mitochondrial antigens. We also sought to explore whether extracellular mitochondria could be mediators of platelet activation in patients with PMR.
Methods: Levels of TSP-1, fMET, and anti-mitofusin 1 IgG (anti-MFN1) were measured by ELISA in the plasma of healthy controls (HC, n=30) and PMR patients before (n=54) and after (n=60) treatment with glucocorticoids. Ultrapure mitochondria isolated from HepG2 cells were opsonized with patient (n=56) or healthy control (n=10) plasma and, upon washing, incubated with platelets isolated from a healthy donor in the presence of the fibrin polymerization inhibitor, GPRP. Mitochondria-mediated platelet activation was assessed by measurement of the platelet cell surface marker P-selectin via flow cytometry.
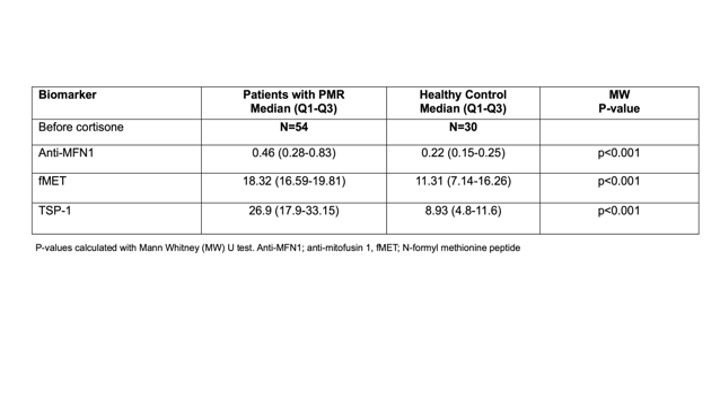
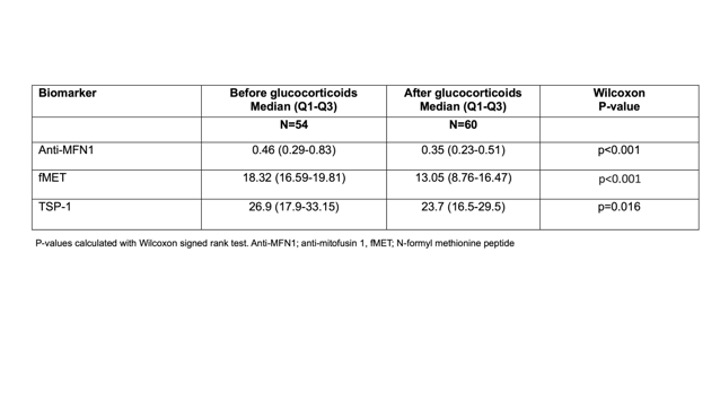
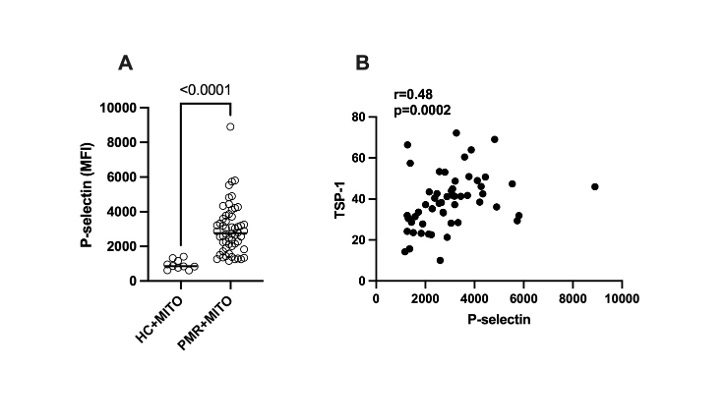
Results: Plasma levels of anti-MFN1 IgG, fMET, and TSP-1 were elevated in patients with PMR before glucocorticoid therapy compared to HC (p< 0.001, Table 1). Levels of anti-MFN1 IgG, fMET, and TSP-1 significantly decreased after treatment with glucocorticoids (p< 0.001, p< 0.001, and p=0.016 respectively, Table 2). Mitochondria opsonized with plasma factors from PMR patients showed markedly higher platelet activation than mitochondria opsonized with plasma factors from healthy individuals (p< 0.0001) (Figure 1A). Further, the extent of platelet activation (P-selectin level) by opsonized mitochondria correlated with plasma levels of TSP-1 in patients with PMR (r=0.48, p=0.0002, Figure 1B). TSP-1 levels were also associated with levels of immune complexes (IC) before glucocorticoid therapy. (r=0.32, p< 0.05).
Conclusion: Our results indicate increased platelet activation, the presence of mitochondrial damage-associated molecular patterns (DAMPs), and AMAs in the circulation of patients with PMR, associated with active disease. These results suggest a pathogenic involvement of mitochondrial antigens and autoantibodies in PMR. Blocking mitochondrial-mediated platelet activation may reduce inflammation in patients with PMR, with potential therapeutic implications.
To cite this abstract in AMA style:
Michailidou D, Johansson L, Armando Gonzalez-Chapa J, Wang T, Chen J, López J, Rantapää-Dahlqvist S, Lood C. Mitochondria-mediated Platelet Activation in Polymyalgia Rheumatica [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/mitochondria-mediated-platelet-activation-in-polymyalgia-rheumatica/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mitochondria-mediated-platelet-activation-in-polymyalgia-rheumatica/