Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Rheumatoid Arthritis – Etiology and Pathogenesis (0795–0800)
Session Type: Abstract Session
Session Time: 2:15PM-2:30PM
Background/Purpose: One of the most substantial impacts of microbe-host interactions at the intestinal mucosa is the development of type 17 immunity. A Th17 signature has been observed in pre-clinical RA, suggesting that type 17 immunity may play a role in the initiation of pre-clinical autoimmunity. Yet, the mechanistic connection between RA-associated bacteria and Th17 immunity remains a knowledge gap. Our prior work in the collagen-induced arthritis (CIA) model of inflammatory arthritis connected microbial dysbiosis with the catabolism of dietary tryptophan to indole, which was necessary for disease via Th17 immunity and pathogenic autoantibody development. In this study, we aimed to examine the mechanism by which indole stimulates Th17 immunity.
Methods: Indole-CIA was induced in male 6-8-week old DBA/1 mice fed a tryptophan-low diet with every-other-day indole gavage (200μl of 10mM) and injected intradermally on days 0 and 21 with type II bovine (CII) emulsified in complete Freund’s adjuvant. Mice also received intraperitoneal injections of 100μg/mouse either isotype control or anti-IL-23 neutralizing antibody once weekly at days 0, 7, 14, and 21 of CIA. Arthritis severity, immune cells, cytokines, and anti-CII antibodies were evaluated by flow cytometry and ELISA. Murine bone marrow–derived dendritic cells (BMDCs) and human gut-like dendritic cells (GDCs) from peripheral blood mononuclear cells were stimulated in vitro with 1μg/ml dose LPS plus 1μl DMSO or LPS plus 1 mM indole for 8-24 hours. The expression of cytokines was measured by qPCR. Signaling pathways were interrogated by RNA sequencing and immunoblot.
Results: IL-23 neutralization, compared to control antibody, significantly reduced the severity of indole-CIA, diminished Th17 cell and DC frequencies, and decreased CII autoantibodies (Fig 1). Upon ex vivo stimulation of splenocytes with CII or anti-CD3/CD28 antibodies, the anti-IL-23 treatment group exhibited significantly lower production of IL-17 and IL-22 compared to the control group (Fig 1). DCs produced Th17-differentiating cytokines at days 7-14 of indole-CIA. In vitro, qPCR analysis showed that indole significantly amplified LPS-induced expression of IL-23, IL-6, IL-1β, and TNF-α in both BMDCs and human GDCs (Fig 2). RNA sequencing of indole-stimulated GDCs followed by immunoblot analysis suggested that indole signaled through non-canonical pathways of aryl hydrocarbon receptor (AhR).
Conclusion: These findings suggest that microbiota-derived indole promotes CIA via priming of intestinal DCs to produce Th17-differentiating cytokines. Non-canonical signaling through AhR results in increased DC production of cytokines that are likely to induce Th17 cells. Blockade of Th17 cell differentiation by IL-23 neutralizing antibody resulted in significantly reduced disease through reduced Th17 cells producing IL-17 and IL-22. Reduction in IL-22 is likely the cause for reduced CII antibodies, although this has yet to be formally tested. Altogether, our data mechanistically connect microbial dysbiosis to the development of inflammatory arthritis, demonstrating one mechanism by which altered microbial ecology can lead to RA.
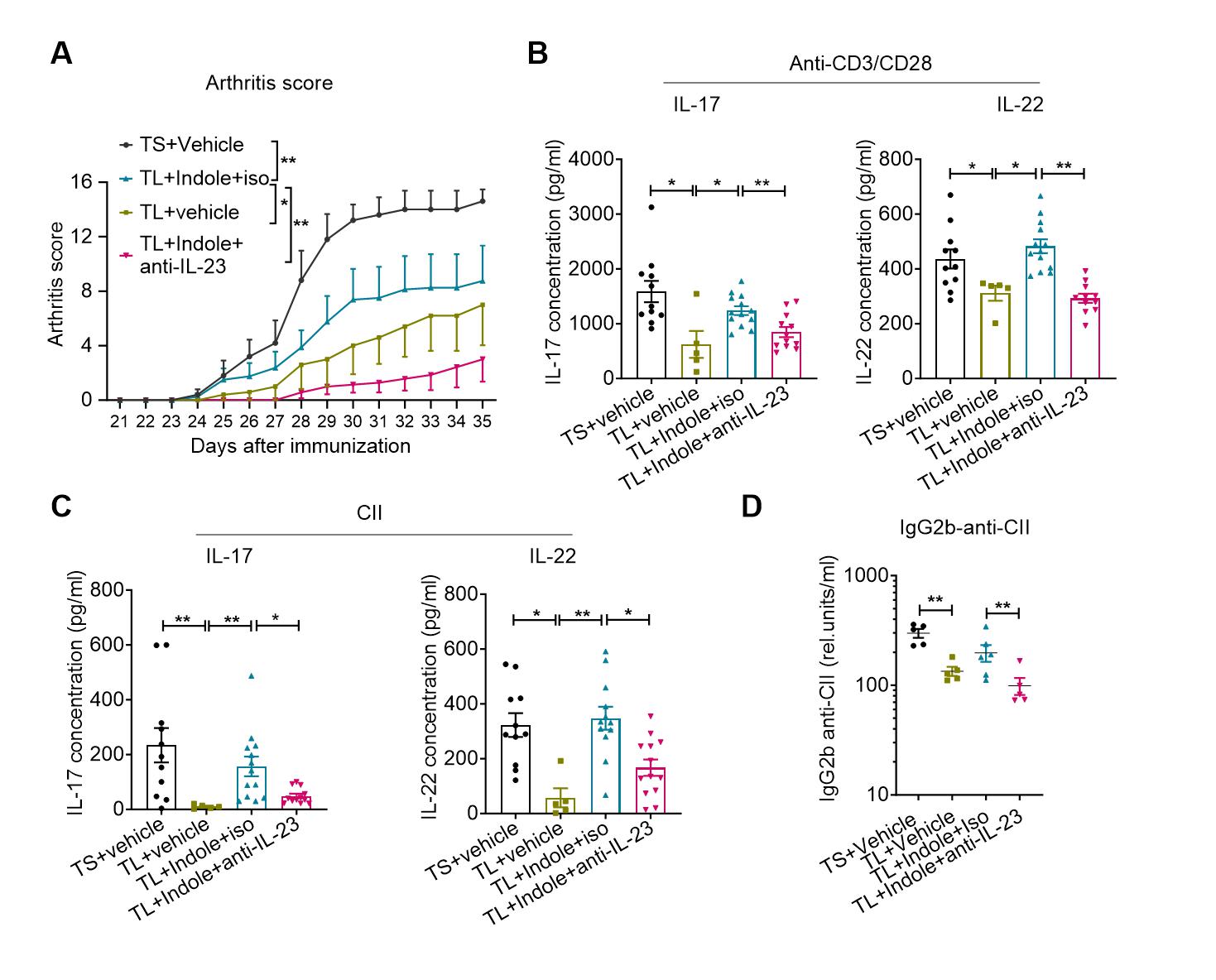 Figure 1. IL-23 neutralization suppresses indole-induced Th17 responses and autoantibodies. CIA was induced in 6–8-week-old male DBA/1 mice maintained on a tryptophan-low (TL) diet, followed by oral gavage of indole (200 μl of 10 mM, every other day). Mice were immunized intradermally on days 0 and 21 with type II bovine collagen (CII) emulsified in complete Freund’s adjuvant (CFA). In addition, mice received intraperitoneal injections of either an isotype control antibody or anti–IL-23 neutralizing antibody (100 μg/mouse) once weekly on days 0, 7, 14, and 21. (A) Neutralization of IL-23 significantly reduced arthritis scores in indole-treated CIA mice compared to isotype control. (B) Ex vivo stimulation of splenocytes with anti-CD3/CD28 antibodies or (C) CII showed reduced production of IL-17 and IL-22 in the anti-IL-23 group, as measured by ELISA. (D) Serum levels of CII-specific IgG2b antibodies were also decreased in the anti–IL-23–treated group compared to controls. Data were expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test (A), or Mann-Whitney test (B-D). *P < 0.05, **P < 0.01, ns: non-significant.
Figure 1. IL-23 neutralization suppresses indole-induced Th17 responses and autoantibodies. CIA was induced in 6–8-week-old male DBA/1 mice maintained on a tryptophan-low (TL) diet, followed by oral gavage of indole (200 μl of 10 mM, every other day). Mice were immunized intradermally on days 0 and 21 with type II bovine collagen (CII) emulsified in complete Freund’s adjuvant (CFA). In addition, mice received intraperitoneal injections of either an isotype control antibody or anti–IL-23 neutralizing antibody (100 μg/mouse) once weekly on days 0, 7, 14, and 21. (A) Neutralization of IL-23 significantly reduced arthritis scores in indole-treated CIA mice compared to isotype control. (B) Ex vivo stimulation of splenocytes with anti-CD3/CD28 antibodies or (C) CII showed reduced production of IL-17 and IL-22 in the anti-IL-23 group, as measured by ELISA. (D) Serum levels of CII-specific IgG2b antibodies were also decreased in the anti–IL-23–treated group compared to controls. Data were expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test (A), or Mann-Whitney test (B-D). *P < 0.05, **P < 0.01, ns: non-significant.
.jpg) Figure 2. Indole enhances LPS-induced Th17-inducing cytokine expression in human GDCs. CD14⁺ monocytes were isolated from peripheral blood mononuclear cells obtained from 10 healthy donors (5 male, 5 female). Cells were cultured in RPMI medium supplemented with 20 ng/ml recombinant human GM-CSF and IL-4 to generate GDCs. On day 3, retinoic acid (100 nM) was added to the culture. On day 5, GDCs were harvested and stimulated with the indicated stimuli for 8 hours. RNA was then extracted, and gene expression was analyzed by qPCR. Results showed that indole significantly enhanced the LPS-induced expression of IL-6, IL-1, and TNF in human GDCs. Data were expressed as mean ± SEM. Statistical significance was determined by Wilcoxon test (A-C). **P < 0.01.
Figure 2. Indole enhances LPS-induced Th17-inducing cytokine expression in human GDCs. CD14⁺ monocytes were isolated from peripheral blood mononuclear cells obtained from 10 healthy donors (5 male, 5 female). Cells were cultured in RPMI medium supplemented with 20 ng/ml recombinant human GM-CSF and IL-4 to generate GDCs. On day 3, retinoic acid (100 nM) was added to the culture. On day 5, GDCs were harvested and stimulated with the indicated stimuli for 8 hours. RNA was then extracted, and gene expression was analyzed by qPCR. Results showed that indole significantly enhanced the LPS-induced expression of IL-6, IL-1, and TNF in human GDCs. Data were expressed as mean ± SEM. Statistical significance was determined by Wilcoxon test (A-C). **P < 0.01.
To cite this abstract in AMA style:
Jing Li J, Liu S, Seymour B, Allen B, Yiphyo L, Levens C, Kuhn K. Microbiota-Derived Indole Promotes Collagen-Induced Arthritis Type-17 Immunity through Intestinal Dendritic Cell Cytokine Production [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/microbiota-derived-indole-promotes-collagen-induced-arthritis-type-17-immunity-through-intestinal-dendritic-cell-cytokine-production/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/microbiota-derived-indole-promotes-collagen-induced-arthritis-type-17-immunity-through-intestinal-dendritic-cell-cytokine-production/
