Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Metabolomics may provide information about the activity and severity of specific diseases and potentially help discriminate between diseases. Choosing the right biological therapy earlier in the course of rheumatoid arthritis (RA) could help to reach the goal of remission. We hypothesized that characterization of patients’ metabolic profiles, utilizing high resolution 1H-nuclear magnetic resonance (NMR), may predict response to tocilizumab therapy prior to treatment in patients with RA.
Methods: 41 patients meeting the 2010 ACR/EULAR classification criteria had initiated treatment with 8 mg/kg of tocilizumab every 4 weeks following usual clinical practice were included in the study. Clinical outcomes were determined at baseline and at 6 months after treatment. Using EULAR response criteria, patients were categorized as responders (good response) or non-responders (moderate response and no response) . Blood was collected at baseline and at 6 months after tocilizumab therapy. A 600 MHz Bruker Avance III spectrometer 1H-NMR was used to acquire NMR spectra of serum samples. Software Chenomx NMR suite 8.5 professional was used for metabolite identification and quantification. Significantly metabolites were identified and the relationship between metabolites and clinical outcome was studied. SPSS and MetaboAnalyis 4.0 KEGG 2019 were used for statistical and pathway analysis.
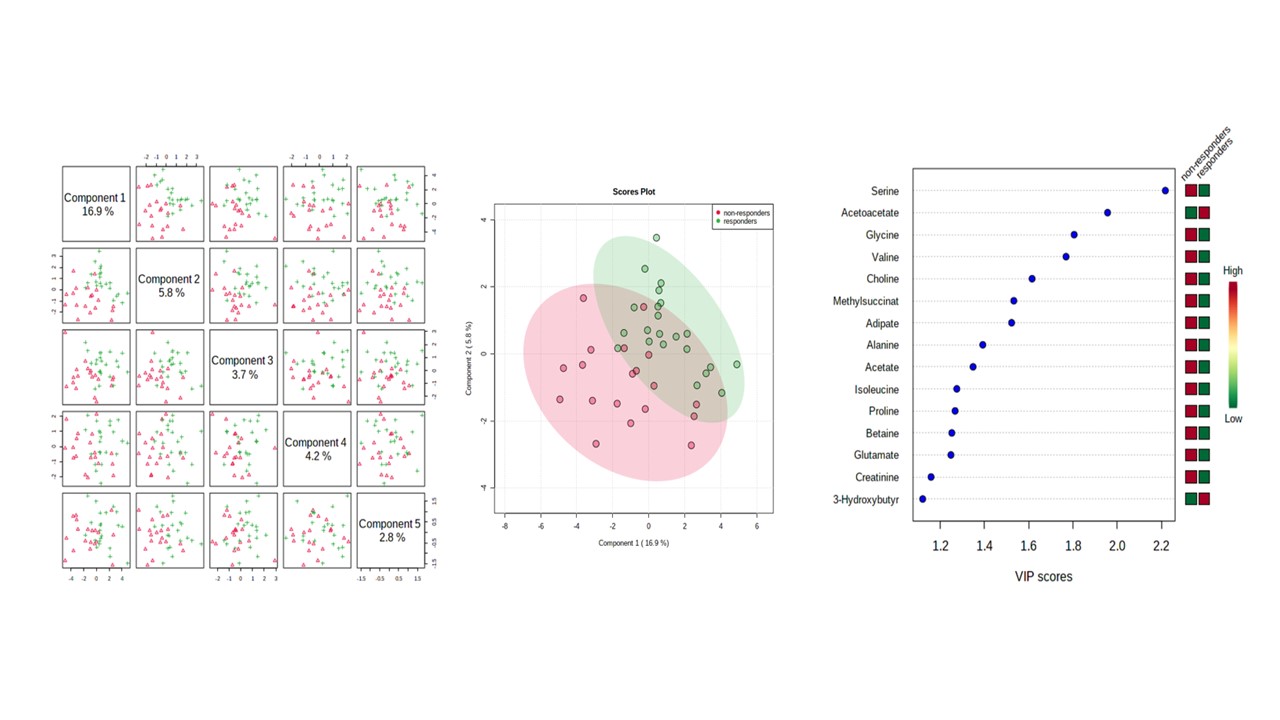
Results: 41 patients (average age 53 , standard deviation (SD) 11) were analyzed. The patients had an initial DAS28CRP of 5.7±1.1. Multivariate statistical analysis of the 1H-NMR baseline spectra successfully discriminated between RA patients classified as tocilizumab responders (n = 22) and non-responders (n = 19), at baseline and 6 months after tocilizumab treatment. A two sample t-test produced p-values of less than 0.05 for seven metabolites which were increased significantly at baseline in responders: 3-hydroxybutyrate, Isobutyrate, Lysine, O-phosphocholine, Phenylalanine, Tryptophan, and Tyrosine. Several metabolites varied their levels following treatement with tocilizumab in both responders and non responders. Of interest, acetoacetate and acetone significantly decreased in non-responders at 6 months of treatment. A PLS-DA (explaining 16.9% of the total variance) confirmed that metabolites profiles differ among groups, although a certain overlap existed (Figure 1). A total of 15 metabolites had VIP scores >1. Individual metabolites yielded area under the curve (AUC) values lower than 70%, indicating relatively poor specificity and sensitivity. A multivariate diagnostic model, showed that concentrations of hydroxybutyrate, leucine, tryptophan, and alanine, improved the sensitivity and specificity of the diagnosis with an AUC of 92.7%. Using this biomarker model, 85% of the patients were correctly classified.
Conclusion: The clear relationship between blood profiles and patient response to tocilizumab therapy suggests that the application of 1H-NMR profiling is a promising clinical tool for RA therapy optimization. More metabolic profiles studies are needed to determine if metabolic profiling can predict response to other biological therapies in RA patients.
To cite this abstract in AMA style:
Murillo-Saich J, Diaz-Torne C, Ortiz M, Coras R, Kavanaugh A, Corominas H, Vidal S, Guma M. Metabolomics Profiling Predicts Outcome of Tocilizumab in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/metabolomics-profiling-predicts-outcome-of-tocilizumab-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/metabolomics-profiling-predicts-outcome-of-tocilizumab-in-rheumatoid-arthritis/