Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Juvenile localised scleroderma (jLS) is a rare rheumatic disease in children characterized by inflammation and fibrosis in the skin [1, 2]. The cause and pathogenesis of jLS remain unclear, and both skin lesions and possible extracutaneous involvement may result in functional impairment and growth disturbances [2]. The treatment options to cure jLS are limited [3].
In recent years, omics technologies have been used to identify novel biomarkers in different diseases [4, 5]. Among the different omics technologies, metabolomics and lipidomics provide snapshots of the metabolic network.
In the current study, we aim to identify biomarkers and treatment targets for jLS using metabolomics and lipidomics.
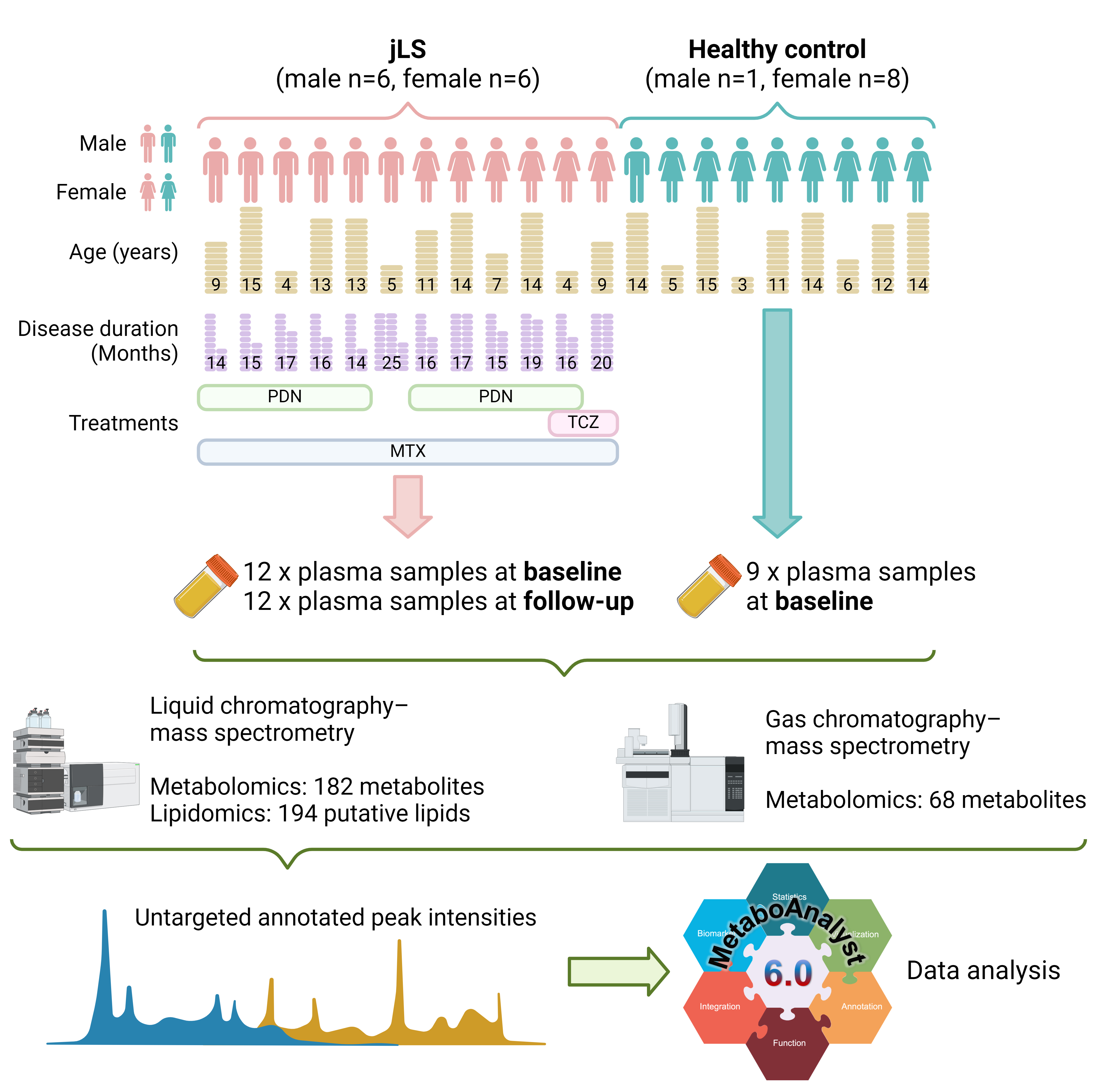
Methods: Children with jLS and age-matched controls were recruited at Bambino Gesù Children’s Hospital, Roma, Italy. The characteristics of the participants are shown in Figure 1.
Plasma samples were collected from 12 children with jLS at baseline (before treatment initiation) and at follow-up (mean duration 17 months after treatment). Nine healthy controls also donated plasma samples at baseline. The samples were sent to Swedish Metabolomics Center, where liquid chromatography–mass spectrometry (LC-MS) and gas chromatography–mass spectrometry (GC-MS) were performed (Figure 1). Peak intensities were recorded, and the data analysis was performed using Metaboanalyst 6.0 and Graphpad Prism 10 software. Pathway enrichment bar plots were generated using SRplot [6]. Mann-Whitney test was used to compare healthy control and baseline patient groups, and Wilcoxon test was used to compare differences between baseline and treated patients.
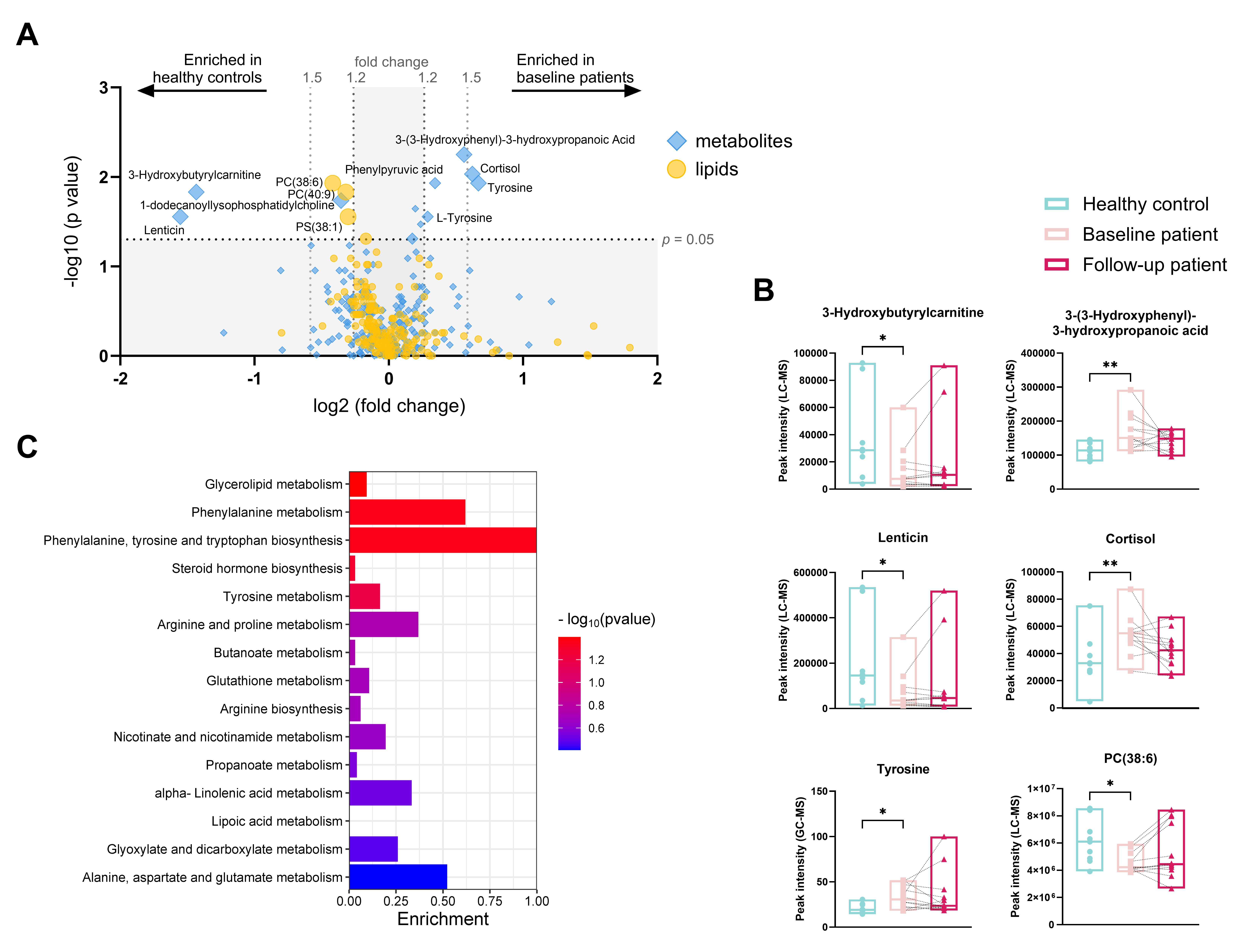
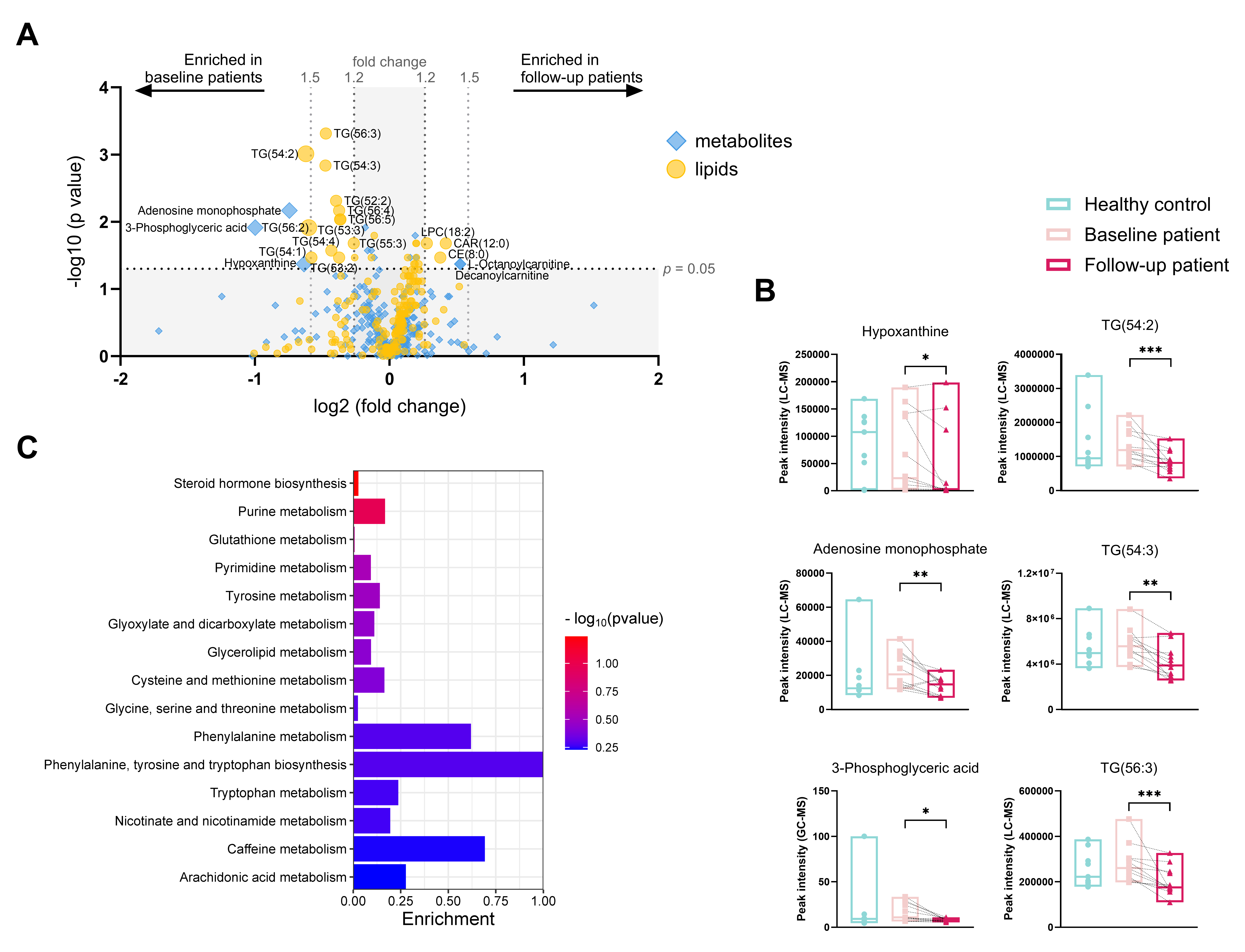
Results: In total, 250 metabolites (182 with LC-MS and 68 with GC-MS) and 194 putative lipids (with LC-MS) were annotated (Figure 1A). Children with jLS at baseline had significantly lower peak intensities of lenticin, 3-hydroxybutyrylcarnitine, 1-dodecanoyllysophosphatidylcholine, phosphatidylcholine (PC) 38:6 and 40:9, and phosphatidylserine (PS) 38:1 as well as significantly higher peak intensities of L-tyrosine, phenylpyruvic acid, 3-(3-Hydroxyphenyl)-3-hydroxypropanoic acid, and cortisol compared to healthy controls (Figure 2A and B). After treatment, peak intensities of adenosine monophosphate, hypoxanthine, 3-phosphoglyceric acid, lysophosphatidylcholine (LPC) 18:2, Cholesteryl Octanoate (CE 8:0), and 2-Hydroxylauroylcarnitine (CAR 12:0) were decreased, whereas peak intensities of L-octanoyl carnitine and eleven molecular species of triacylglycerols were increased compared to the baseline group (Figure 3A and B). The top affected pathways were shown in Figure 2C and 3C.
Conclusion: We have described the metabolic profile in the blood of children with jLS for the first time. Children with jLS showed a distinct metabolic profile compared to healthy children, especially in tyrosine-related pathways. Compared to baseline levels, the metabolism of several amino acids was altered after treatment, and the energy storage function might be modified as eleven molecular species of triacylglycerols were found decreased.
To cite this abstract in AMA style:
Zhang Y, Aquilani A, Nicolai R, De Benedetti F, Marasco E, Maglio C. Metabolomics and Lipidomics in Juvenile Localized Scleroderma [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/metabolomics-and-lipidomics-in-juvenile-localized-scleroderma/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/metabolomics-and-lipidomics-in-juvenile-localized-scleroderma/