Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: While BMI has been associated with response to therapy in several studies in rheumatoid arthritis (RA), it is not a comprehensive measure of the metabolic consequences of obesity. Few studies have evaluated metabolic syndrome (central adiposity, hypertension, lipid abnormalities, and insulin resistance), its individual components, and their independent effects on treatment response. We determined if metabolic syndrome, its components, and adipokines (adiponectin, leptin, Fibroblast Growth Factor-21) were associated with response to biologic therapies among patients with RA.
Methods: This study included participants with RA and at least moderate disease activity initiating either TNFi or non-TNFi biologic therapies from the Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions (CERTAIN) cohort within the CorEvitas registry. Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III definition. Adipokines were assessed on stored samples from a sub-sample of responders and non-responders to both TNFi and non-TNFi therapies (N=200). The primary outcome was the achievement of a change as large as the minimal clinically important difference (MCID) for the Clinical Disease Activity Index (CDAI) at 6 months. Low disease activity was a secondary outcome (CDAI< 10). Associations were quantified using multivariable logistic regression, accounting for age, sex, baseline CDAI, prior biologic use, biologic class, ACPA/RF status, disease duration, and CRP.
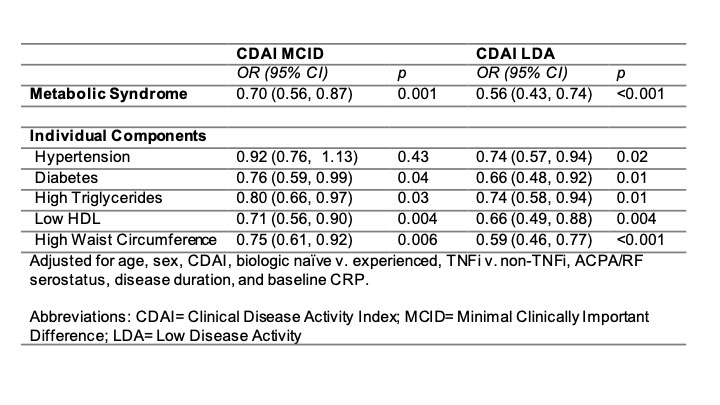
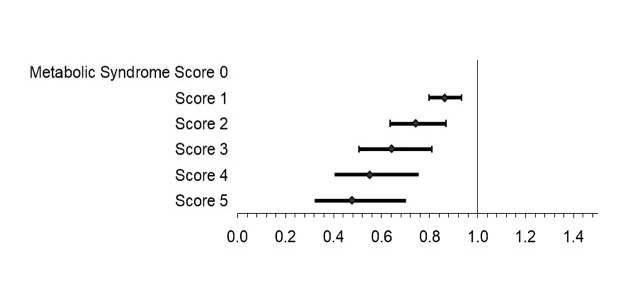
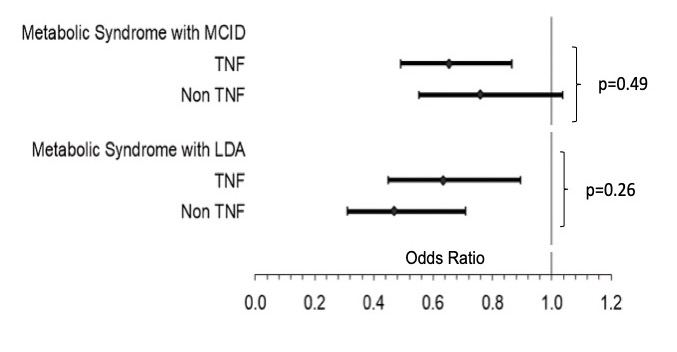
Results: Among 2,368 participants, 687 (29%) had metabolic syndrome. Metabolic syndrome was associated with lower odds of achieving CDAI MCID [OR: 0.69 (95% CI: 0.56,0.86) p=0.001]. Each component except hypertension was associated with a lower odds of achieving MCID and each was associated with low disease activity (LDA) (Table 1). There was a dose-dependent reduction in response rate according to the number of components present (Figure 1). The addition of metabolic syndrome to a model that included BMI improved model fit (p=0.008). Only underweight BMI and severe obesity were associated with a lower odds of response compared to normal BMI independent of metabolic syndrome [OR (underweight): 0.24 (95% CI: 0.075,0.77), p=0.02]. Associations between metabolic syndrome and response were similar between patients receiving TNFi [OR: 0.65 (95% CI: 0.49,0.87) p=0.003] v. non-TNF therapies [OR: 0.76 (95% CI: 0.55,1.04), p=0.08] (p-for-interaction=0.49) (Figure 2). Adipokines were each associated with the presence of metabolic syndrome but were not associated with the achievement of MCID.
Conclusion: Metabolic syndrome is associated with lower response rates with the initiation of a biologic therapy in RA, with similar effects for both TNFi and non-TNFi agents. These observations suggest that metabolic aspects of obesity, independent of weight alone, are important. Adipokines were associated with metabolic syndrome but not with clinical response suggesting they do not play a mediating role. These associations have implications for trial design and clinical practice, though further study is needed to define the underlying mechanisms.
To cite this abstract in AMA style:
Baker J, Reed G, Thiele G, Pappas D, Charles-Schoeman c, Guma M, Harrold L, Curtis J, Kremer J. Metabolic Syndrome, Adipokines, and Response to Advanced Therapies in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/metabolic-syndrome-adipokines-and-response-to-advanced-therapies-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/metabolic-syndrome-adipokines-and-response-to-advanced-therapies-in-rheumatoid-arthritis/