Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: B cell dysregulation and production of autoantibodies against autoantigens are hallmarks of Systemic lupus erythematosus (SLE). In healthy adults (HC), B cells with autoreactive antibodies are eliminated from the repertoire before they reach the B cell maturation stage, a mechanism that is altered in SLE.
Methods: In order to characterize the dysregulated B cells in SLE, we performed single-cell RNA-sequencing and BCR repertoire-sequencing of B cells from SLE patients (n=10) and healthy controls (n=10). Further, we performed informatic analyses to define dysregulated transcriptional pathways associated with B cell subsets is SLE as compared to healthy subjects. We expressed monoclonal antibodies (mAbs) encoded by the clonally expanded and dysregulated B cells in SLE (n=100) and screened their antigens specificity using custom Luminex array that include SLE autoantigens and viral proteins.
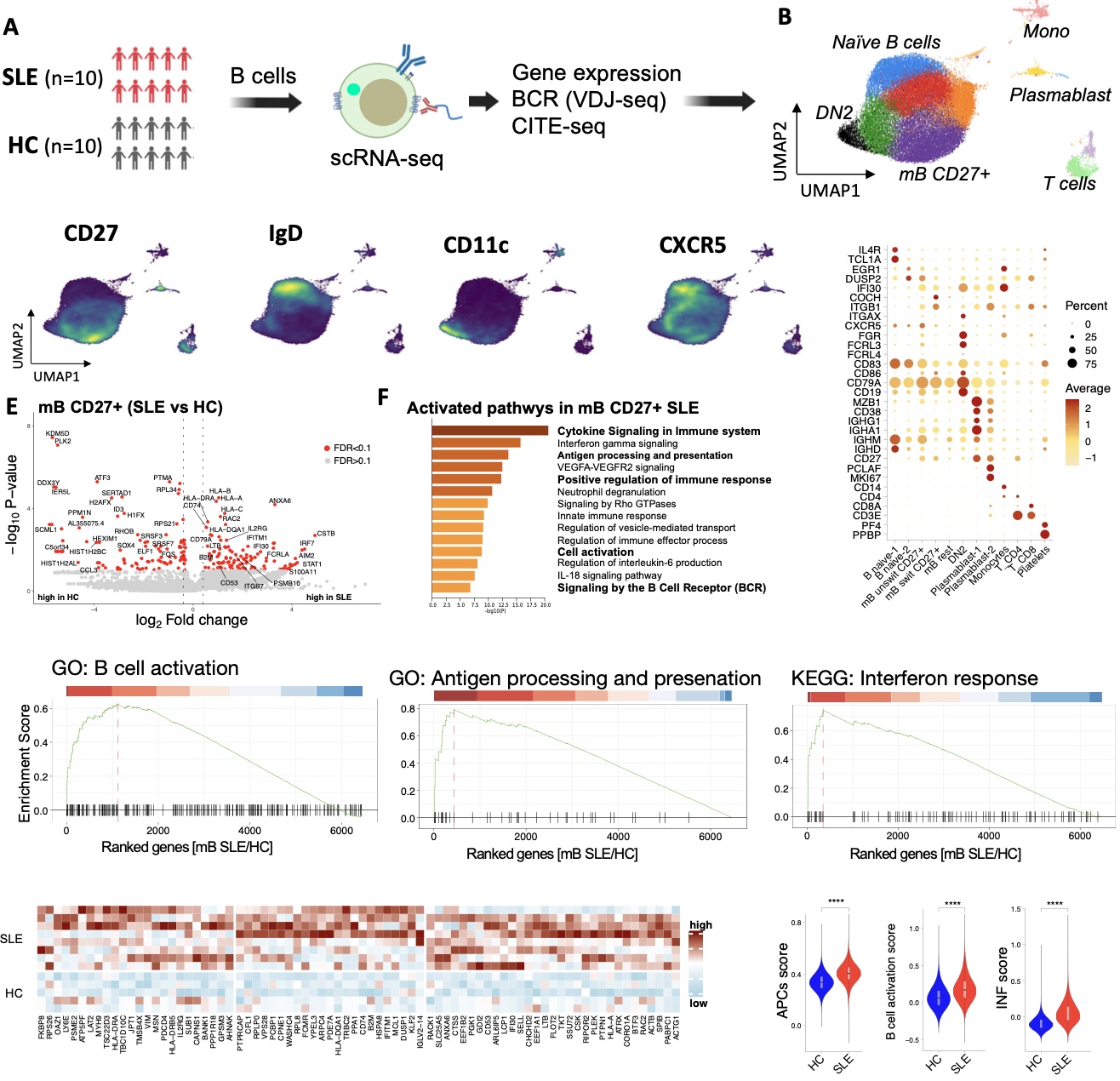
Results: Single cell RNA sequencing followed by unsupervised clustering defined subsets of B cells representing the naïve, memory and plasmablasts populations (Fig 1A and B). To elaborate on the subset of memory B (mB) cells, we further annotated them into CD27+ mB cells (IgD- CD27+ CD11c-) and double negative (DN2) mB cells (IgD- CD27- CD11c+, Fig. 1C and D). Transcriptome analyses of CD27+ mB B cells derived from SLE revealed remarkable upregulation of genes involved in B cell activation as well as antigen processing and presentation pathways, as compared to healthy CD27+ mB cells (Fig. 1E and F). Among those upregulated genes are IFI30, CD74, CD53, HLA-DRA and IL2RG (Fig. 1G and H).
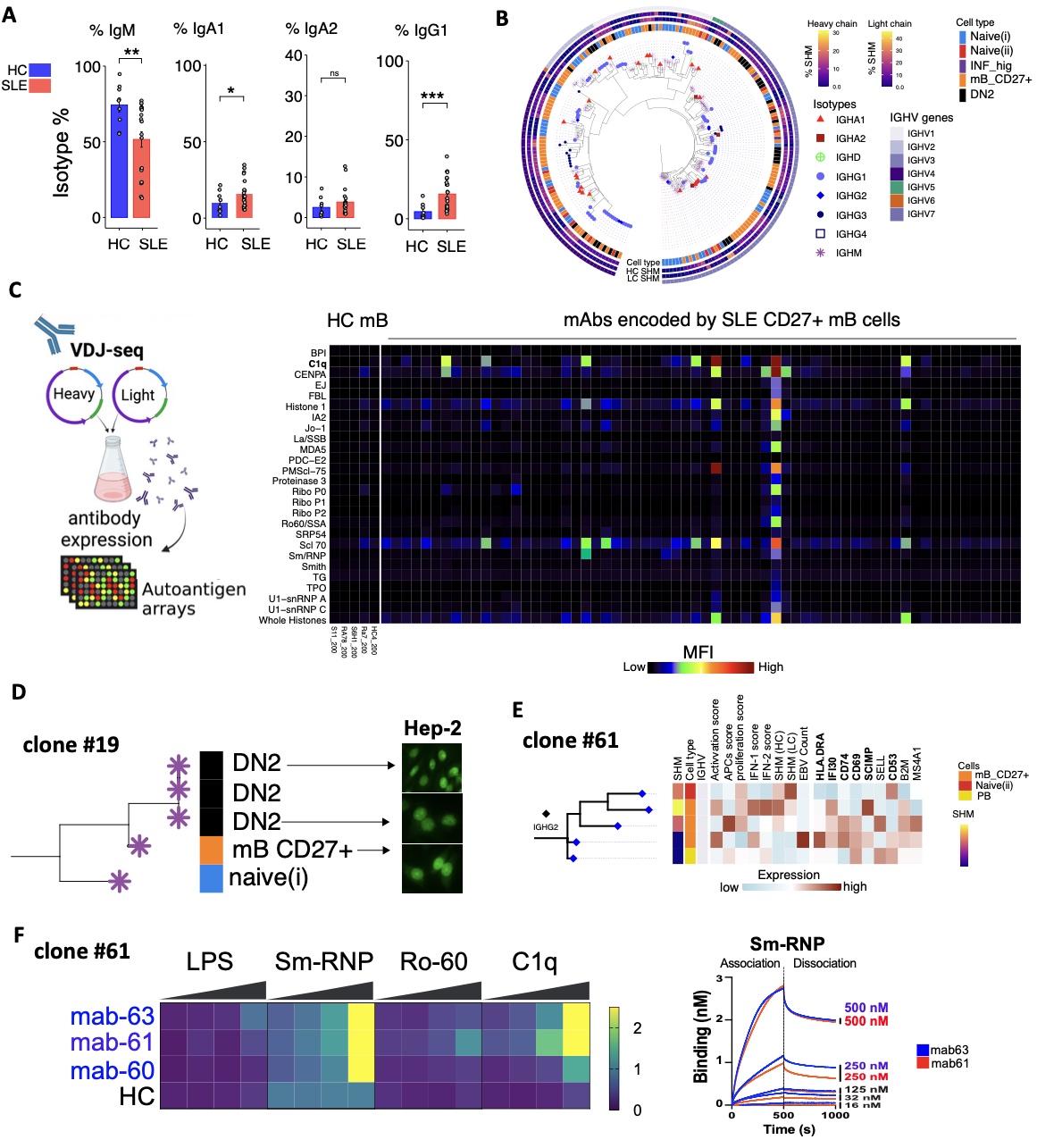
BCR analysis of sequenced SLE B cells showed that the CD27+ mB and DN2 cells are class-switched and have increased somatic hypermutations (SHM) of their BCR’s heavy and light chains, compared to HC (Fig2 A). The largely expend clones in SLE with highest SHM rates belongs to CD27+ mB compartment (Fig2 B). Characterization of mAbs encoded by clonally expanded and dysregulated B cells in SLE identified multiple SLE B cell mAbs that stained the HEP-2 cell line and bound self-antigens on antigen arrays, including anti-C1q, anti-Sm-RNP, anti-Ro60 and anti-dsDNA mAbs (Fig. 2C -G).
Conclusion: This study characterized dysregulated transcriptional pathways associated with B cell subsets is SLE as compared to healthy subjects, including of clonally expanded B cells. Our findings revealed a subset of dysregulated CD27+ memory B cells encoding anti-nuclear autoantibodies in SLE and exhibited elevated B cell activation and antigen processing and presentation pathways. Multiple studies have reported the pathogenic role of DN2 cells in SLE and other autoimmune diseases as the source of autoantibodies. We demonstrate that clonally expanded autoimmune B cells are shared between CD27+ and DN2 linages. Our results suggest that memory B cells undergo cell activation, encode autoantibodies and consequently contribute to the development of pathogenic DN2 B cells.
To cite this abstract in AMA style:
Younis S, Moutusy S, Jahanbani S, Wu X, Harris M, Pandit M, van Dam L, Sharpe o, Utz P, Robinson W. Memory B Cell Activation and Dysregulation in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/memory-b-cell-activation-and-dysregulation-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/memory-b-cell-activation-and-dysregulation-in-systemic-lupus-erythematosus/