Session Information
Date: Sunday, November 17, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In keeping with hallmark clinical evidence of stiffening and fibrotic thickening of the skin, we and others have previously demonstrated a role for the MRTF-A mechano-sensing pathway in the persisting activation of disease myofibroblasts. However, the significance of this pathway in resident immune cells within the diseased tissue remains underexplored. To address this gap, we employ tissue culture modelling on soft and stiff matrices, immunostaining, and the use of generic small molecule inhibitors to investigate the role of MRTF-A signalling in maintaining macrophage activation in SSc.
Methods: Nuclear MRTF-A in Mφ within systemic sclerosis (SSc) tissue was analysed by immunostaining for CD86 and MRTF-A, distinguishing between cytoplasmic and nuclear expression. The effect of substrate stiffness on Mφ was assessed using collagen-coated plates soft (4kPa) and stiff (50kPa) and utilizing monocyte-derived Mφ from both healthy controls (HC) and SSc patients. Cellular expression of MRTF-A in SSc Mφ was confirmed by PCR product analysis on gels, with inhibition achieved via the commercially available compound CCG-1432. The cytokine profile was evaluated using a Luminex-based bead array. Phagocytosis was measured using Red E. coli BioParticles, and MERTK expression in monocyte-derived Mφ was quantified by qPCR.
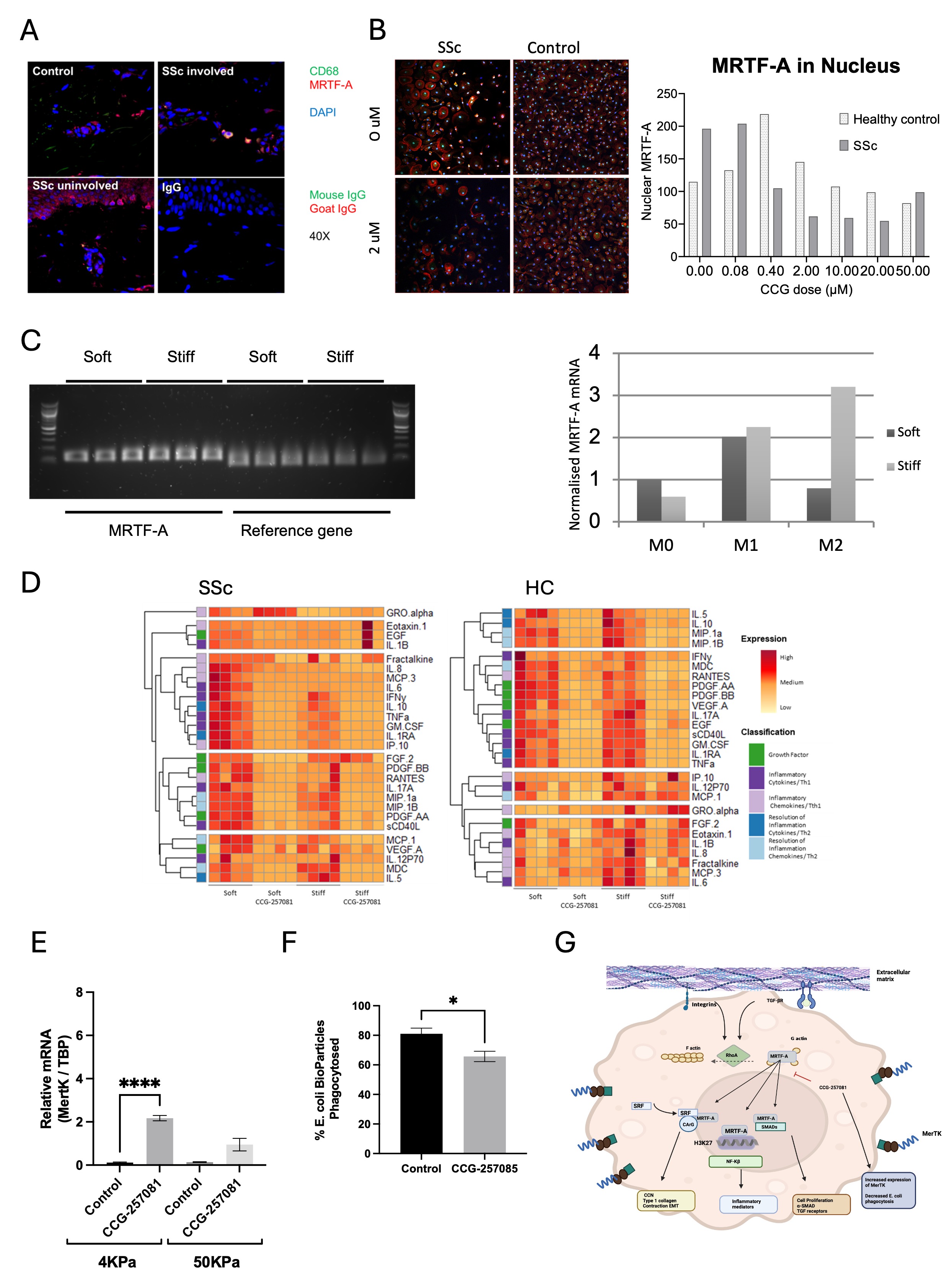
Results: In our study, we identified Mφ within the disease tissue that were positive for MRTF-A in the nuclear compartment (Fig1A&B) and observed expression of MRTF-A mRNA in SSc monocyte-derived Mφ (Fig 1C). Culturing these Mφ on stiff versus soft substrates triggered MRTF-A nuclear translocation and induced cytokine release from HC Mφ. In systemic sclerosis (SSc), high-level cytokine release by Mφ was observed on soft substrates and was not further enhanced by stiff tissue culture (Fig 1D). Specifically, the secretion levels of TNF-α, IL1RA, GMCSF, CXCL1, and CCL7 were significantly decreased (p=0.0047, p=0.0049, p=0.0615, p=0.0004, and p=0.0091, respectively), while no significant changes were observed in HC (p=0.8842, p=0.9380, p=0.9636, and p=0.3601) except for CCL7, which increased in response to stiffness (p=0.0290). Moreover, the CCG drug suppressed MRTF-A translocation on hard substrates (IC50 0.4μM) and blocked the expression of TNF-α, IL-1RA, and GMCSF regardless of stiffness or disease. Functional assays using the CCG drug showed decreased phagocytosis of E. coli particles and an increase in the efferocytosis marker MERTK in SSc(Fig 1E&F).
Conclusion: Our analyses indicate nuclear translocation in SSc Mφ skin and confirm the presence of MRTF-A mRNA in monocyte-derived Mφ. MRTF-A was shown to be translocated to the nucleus in culture on hard substrates, a process that was suppressed by the CCG drug inhibitor. Blockade of MRTF-A reduced the release of multiple cytokines and growth factors, indicating a role for MRTF-A in modulating inflammation independent. Furthermore, inhibition of MRTF-A might modulate macrophage phenotype from inflammatory to regulatory, as evidenced by decreased phagocytosis and increased efferocytosis markers. Therapeutically targeting MRTF-A could potentially modulate pathogenicMφ in SSc
A) Immunostaining of macrophages (CD68) and MRTF-A in SSc skin. B) Immunostaining of MRTF-A translocation and inhibition with CCG_25780. C) MRTF-A mRNA in SSc macrophages in soft and stiff substrates. D) Multiplex cytokine ELISA secreted by macrophages in soft (4kPa) and stiff (50kPa) with and without inhibitor. E) Effect of inhibitor on MertK expression in SSc macrophages. F) E.coli phagocytosis decreases with inhibitor in SSc macrophages. G summary findings of MRTF-A pathway in macrophages and response to CCG drug.
To cite this abstract in AMA style:
Lopez Garces S, Lim G, Liu J, Choudhary M, Vimalenthiran, J, Tam A, Abraham D, Denton C, Ahmed Abdi B, Stratton R. Mechano-transduction via MRTF-A Pathway Is Required for Cytokine Release by Scleroderma Macrophages [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/mechano-transduction-via-mrtf-a-pathway-is-required-for-cytokine-release-by-scleroderma-macrophages/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mechano-transduction-via-mrtf-a-pathway-is-required-for-cytokine-release-by-scleroderma-macrophages/