Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: While remission off medication is the goal in JDM, timely achievement of clinically inactive disease (CID) is an important interim outcome. Data from the CARRA JDM Registry data, a multi-center North American JDM inception cohort, was evaluated to quantify the number of patients who met CID at 12 months. We also assessed baseline characteristics associated with achieving CID.
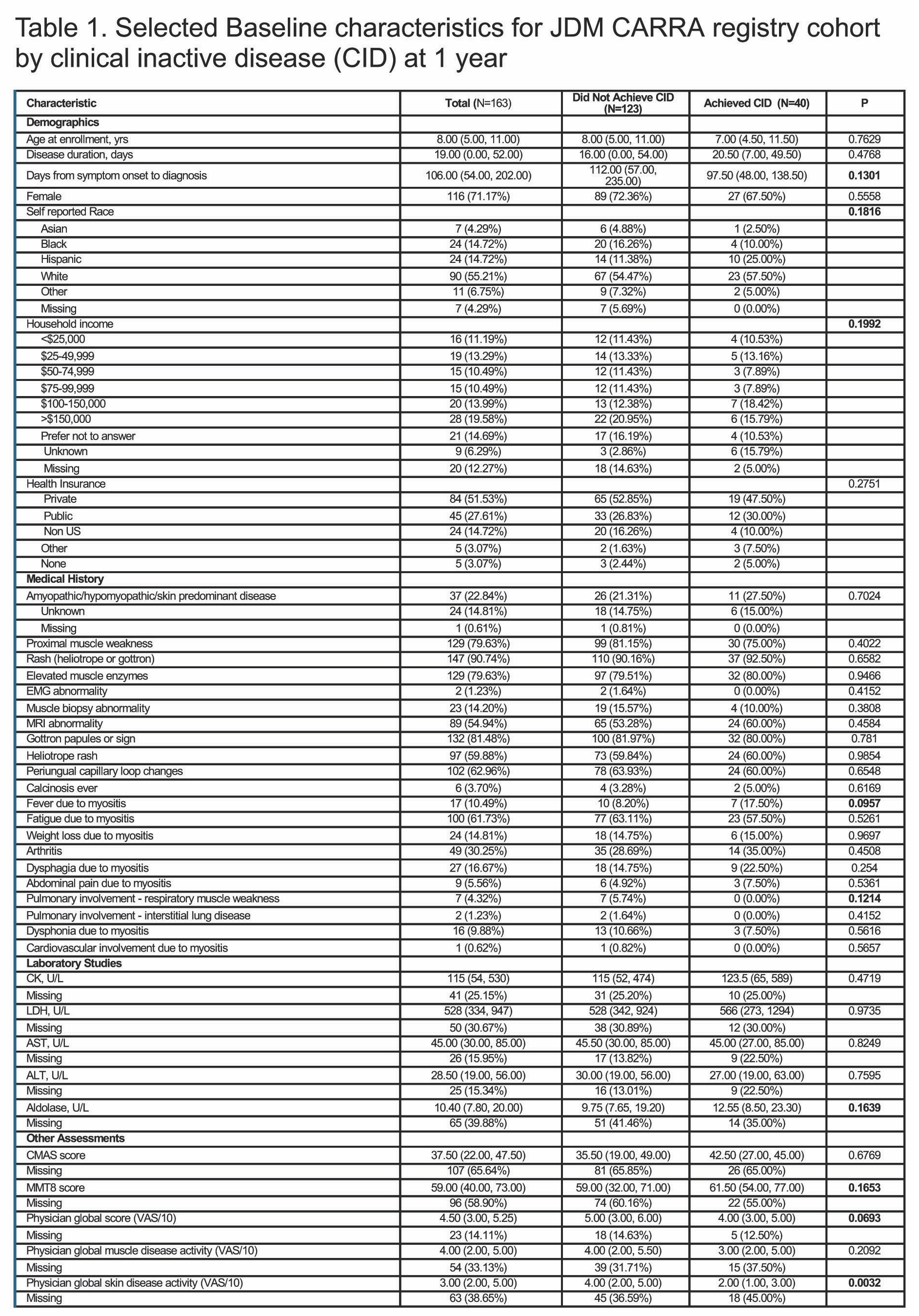
Methods: The CARRA registry JDM inception cohort includes JDM patients within 6 months of diagnosis with less than 12 weeks of systemic treatment. Data from baseline and 12 month (+/- 3 months) follow up visits were analyzed. The primary outcome (CID at 12 months) was defined by modified PRINTO criteria (meet 3/3 criteria: 1) Physician Global Activity visual analog score (VAS) 0/10, 2) creatine kinase (CK) less than 150 or other muscle enzyme elevation if CK missing, 3) Childhood Myositis Assessment Scale (CMAS) 48+/52, Manual Muscle Testing-8 (MMT) of 78+/80, or absence of proximal weakness). Clinical predictors assessed included demographics, clinical features, lab results, disease activity assessments and patient-reported outcomes (Table 1). Univariate analysis to quantify baseline characteristics associated with CID was performed using Wilcoxon rank-sum test or chi-square test. Logistic regression with backward selection of all variables in the univariate analysis with P< 0.2 was performed with missing data imputed.
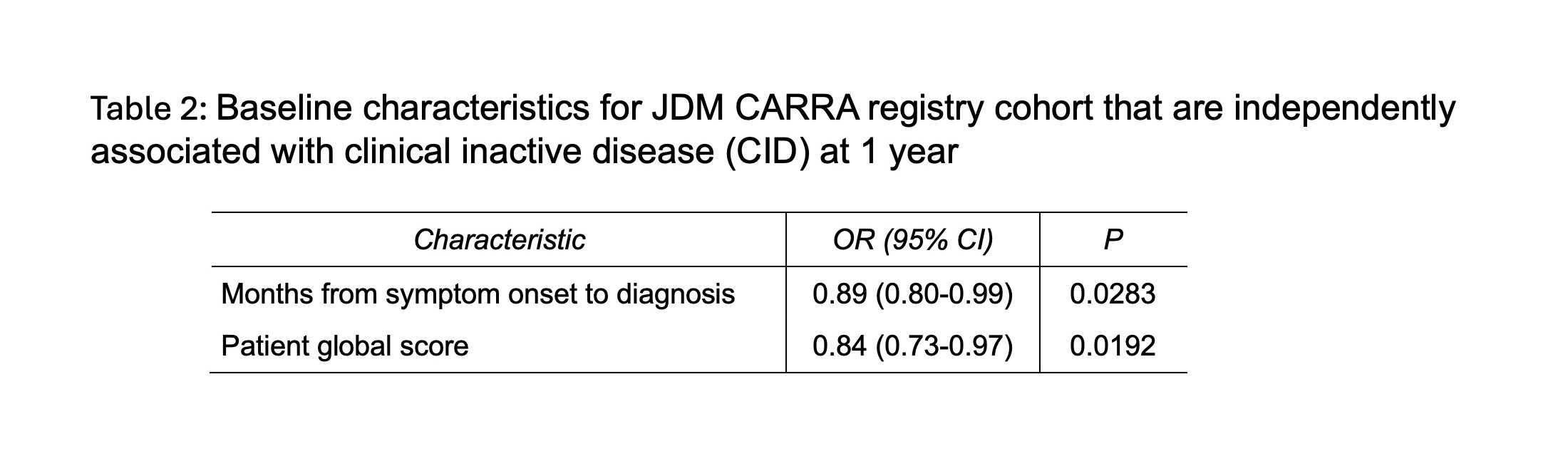
Results: 163/198 (82.3%) patients had complete data on the modified PRINTO criteria at follow-up. 40/163 (24.5%) had CID at 12 months. Key univariate analyses comparing baseline characteristics and disease features from those who achieved CID versus those who did not are shown in Table 1. In unadjusted univariable analysis, those who achieved CID notably had lower patient global activity (VAS) (p= 0.02) and skin disease activity VAS (p=0.003) at baseline. In multivariable modeling, patient global VAS remained significant (p=0.02, OR 0.84, 95%CI 0.73-0.97). The other baseline variable independently associated with CID at 12 months was time from symptom onset to diagnosis, with shorter time increasing odds of CID (p=0.03, OR 0.89, 95% CI 0.80-0.99).
Conclusion: These results highlight that achievement of CID at 12 months in JDM was met only one quarter of the time, providing a benchmark for improving care. These results highlight a need to improve overall achievement of CID, such as by development of targeted therapies to improve disease control in JDM particularly for patients with moderate to severe disease at diagnosis. Patients with mild disease at onset by patient global disease activity, and a shorter time from symptom onset to diagnosis tend to have better outcomes also suggesting a need to decrease time to diagnosis for patients.
Acknowledgements: CARRA, AF, IRP of NIH, NIAMS
CID: clinically inactive disease, EMG: electromyography, CK: creatine kinase, LDH: lactate dehydrogenase, AST: aspartate transaminase, ALT: alanine transaminase, CMAS: Childhood Myositis Assessment Scale, MMT8: Manual Muscle Testing 8, CHAQ: Childhood Health Assessment Scale, PROMIS: Patient-Reported Outcomes Measurement Information System, VAS: visual analog scale out of 10
Univariable analysis was done to quantify baseline characteristics associated with inactive disease at 1 year was performed using a Wilcoxon rank-sum test or chi-square test. All variables with a p-value of <0.2 (bold above) were included in multivariable analysis. CID defined as presence of all 3 modified PRINTO criteria (1) Physician Global Activity visual analog score or VAS 0 /10, 2) creatine kinase ≤150, and 3) Childhood Myositis Assessment Scale (CMAS) ≥48/52 and Manual Muscle Testing-8 (MMT-8) of ≥78/80 or “no proximal weakness” clinically). Continuous traits are listed with median (1st and 3rd quartile). Categorical traits are listed with n (%).
To cite this abstract in AMA style:
Neely J, Shrader P, Tarvin S, Ardalan K, Shenoi S, Huber A, Kim S, Kim H. Measuring Clinically Inactive Disease at One Year in Patients with Juvenile Dermatomyositis (JDM) in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/measuring-clinically-inactive-disease-at-one-year-in-patients-with-juvenile-dermatomyositis-jdm-in-the-childhood-arthritis-and-rheumatology-research-alliance-carra-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/measuring-clinically-inactive-disease-at-one-year-in-patients-with-juvenile-dermatomyositis-jdm-in-the-childhood-arthritis-and-rheumatology-research-alliance-carra-registry/