Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is a disease-specific measure of osteoarthritis (OA) symptoms (pain and stiffness) and functional impairment regularly used in clinical trials. To aid interpretation of the clinical meaningfulness of response to treatment, it is important to determine thresholds for WOMAC that would indicate meaningful within-patient change (MWPC), where an individual has experienced a meaningful clinical benefit. Our aim therefore was to define MWPC in WOMAC by examining the relationship between change in WOMAC domains and change in patient global assessment (PGA-OA) as an anchor (as recommended in recent FDA guidance1) in patients with moderate-to-severe OA.
Methods: WOMAC numerical rating scale (NRS) 3.1 Index2 consists of 24 items measuring pain (5 items), stiffness (2 items) and physical function (17 items), assessed using a 0-10 NRS with higher scores indicating worse outcomes. Data were analyzed separately from three Phase 3 clinical trials of tanezumab, a novel treatment intended for the relief of signs and symptoms of moderate-to-severe OA, administered subcutaneously every 8 weeks. Studies 1 (NCT02697773) and 2 (NCT02709486) were 16- and 24-week placebo-controlled trials, respectively; study 3 (NCT02528188) was a 56-week active-controlled trial. Patients with moderate-to-severe OA of the hip or knee completed the WOMAC and PGA-OA at regular time points. A repeated measures model with change in WOMAC domain score as the outcome and change in PGA-OA as the anchor was used to establish MWPC for WOMAC domains.
Results: In the 3 studies there were 688, 844, and 2948 subjects available for analyses, respectively. Analysis showed that a linearity assumption for the relationship between changes in WOMAC domains and changes in PGA-OA was appropriate. Moreover, the relationships between these changes were very close for two trials and similar for the third (Figure 1). The estimated MWPC for the three WOMAC domains were from 0.8 to 1.2 (NRS from 0 to 10) and from 12.5 to 16.2%, depending on study and domain, that corresponded to a 1- category change on PGA-OA (Table 1). For a 2-category change those values were from 1.7 to 2.3 and from 25.0 to 32.5%, respectively. Values were similar to and supportive of published results.3 While the relationship between change in PGA-OA and change in WOMAC was approximately linear, the correspondence between, for example, ratings of ‘no change’ on the PGA-OA with changes in WOMAC pain may be a manifestation of their measuring similar but distinct concepts.
Conclusion: These results establish MWPCs for WOMAC domains, at the individual patient level, for patients with moderate-to-severe OA of hip or knee. The relationship between change in PGA-OA and change in WOMAC is the focus of ongoing evaluation.
These studies were sponsored by Pfizer and Lilly. Medical writing support was provided by Steven Moore, PhD, of Engage Scientific Solutions and was funded by Pfizer and Lilly.
References
1FDA Patient-Focused Drug Development Guidance Workshop, 6 Dec 2019.
2Bellamy N (2008). WOMAC Osteoarthritis Index: user guide IX.
3Erdogan BD et al (2016). J Rheum 43 (1) 194-202.
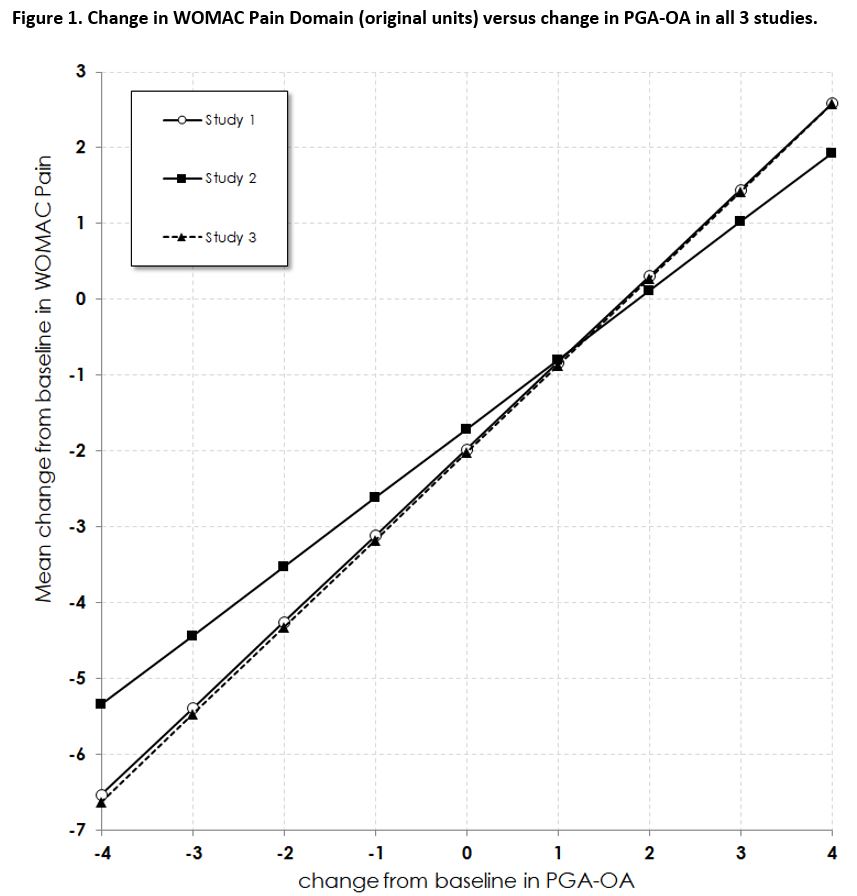 PGA-OA, patient’s global assessment of osteoarthritis; WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
PGA-OA, patient’s global assessment of osteoarthritis; WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
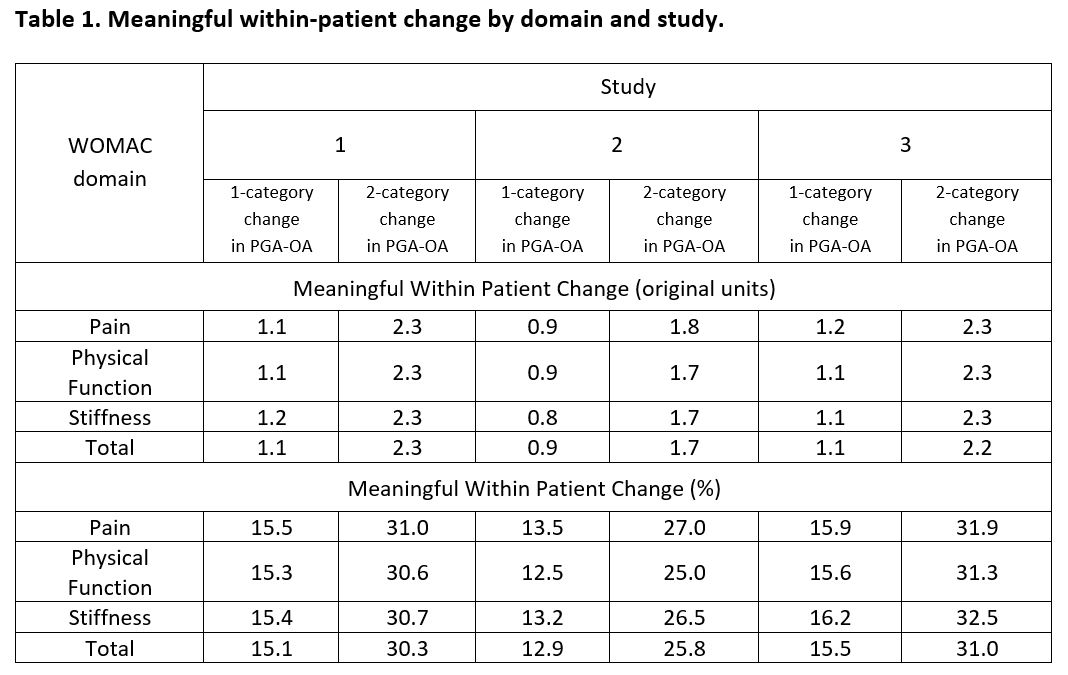 PGA-OA, patient’s global assessment of osteoarthritis; WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
PGA-OA, patient’s global assessment of osteoarthritis; WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
To cite this abstract in AMA style:
Conaghan P, Dworkin R, Schnitzer T, Berenbaum F, Bushmakin A, Cappelleri J, Viktrup L, Abraham L. Meaningful Within-Patient Change in WOMAC Domains in Patients with Moderate-To- Severe Osteoarthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/meaningful-within-patient-change-in-womac-domains-in-patients-with-moderate-to-severe-osteoarthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/meaningful-within-patient-change-in-womac-domains-in-patients-with-moderate-to-severe-osteoarthritis/
