Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The presence of anti-melanoma differentiation associated protein 5 (MDA5) autoantibodies in myositis patients is associated with mucocutaneous ulcerations, (rapidly progressing) interstitial lung disease (RPILD), arthritis and mild muscle involvement. There are no biomarkers to identify patients that will develop RPILD, although early initiation of treatment is essential to rescue patients from respiratory failure. The aetiology is unresolved and it is unknown which domain of the MDA5 protein is the main elicitor of an immunogenic response.
The aim of this study is to identify the domains in the MDA5 protein that are the target of autoantibodies, which could lead to more insight into the disease mechanism.
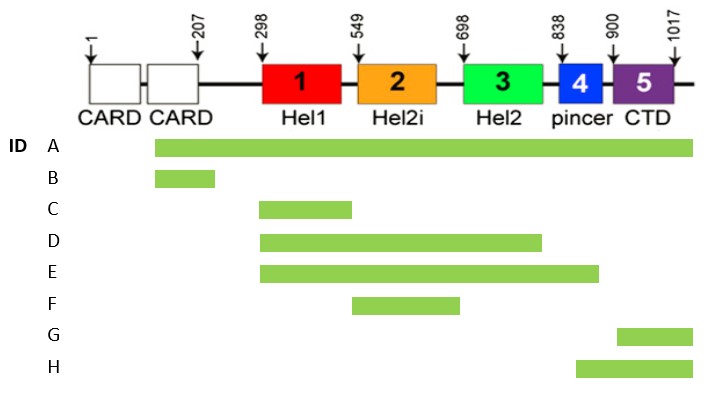
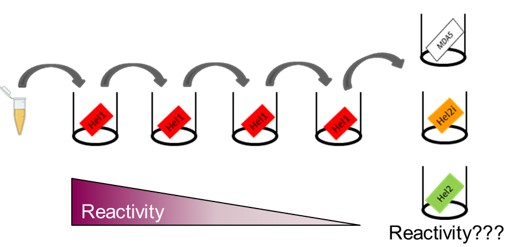
Methods: The reactivity in sera from MDA5+ dermatomyositis patients at baseline (n=20, Karolinska Institutet) and purified IgG were measured in ELISA (OD). IgG were isolated from MDA5+ plasmapheresis samples (n=9, from France, Sweden and Belgium) by affinity chromatography as previously described (1). All target proteins, MDA5 constructs (UniProt ID Q9BYX4, Figure 1) and RIG-I (UniProt ID O95786), were produced in E.coli. A depletion ELISA was developed to assess the specificity of the reactivity towards the different domains. Briefly, the reactivity towards one construct was depleted in several incubation steps before the purified IgG were transferred to another construct to measure the remaining reactivity (Figure 2). The RIG-I protein was used as a negative control.
Results: All MDA5+ patients (n=20) showed reactivity towards the helicase (Hel) domains at the center of the MDA5 protein, but reactivity towards construct B was significantly lower compared to construct A (median OD 0.940 vs 3.13, p< 0.0001) and reactivity towards construct G varied between patients (median OD=2.285 [1.057-3.109]). There was no reactivity towards the RIG-I protein (0/29). After depleting antibodies that target Hel1 (or Hel2i), the reactivity towards Hel2i and Hel2 (or resp. Hel1 and Hel2) persisted.
Conclusion: Anti-MDA5 autoantibodies in plasma of dermatomyositis patients mainly target the helicase domains of the MDA5 protein. Our depletion experiments suggest that patients can have specific autoantibodies towards each helicase domain, which implies that multiple epitopes are present on the MDA5 protein. The absence of reactivity towards construct B possibly suggests the structure of the autoantigen might differ from the endogenous MDA5 protein, but further experiments are necessary. The potential usage of anti-MDA5 reactivity as a biomarker for RPILD will be explored in an extended cohort.
Reference:
(1) Ossipova E, Cerqueira CF, Reed E, Kharlamova N, Israelsson L, Holmdahl R, et al. Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Arthritis Res Ther. 2014;16(4).
To cite this abstract in AMA style:
Van Gompel E, Cerqueira C, Chemin K, Horuluoglu B, Galindo-Feria A, amara k, Wigren E, Gräslund S, De Langhe E, Benveniste O, Lundberg I. MDA5 Helicase Domains Identified as the Main Targets of Anti-MDA5 Autoantibodies in European Dermatomyositis Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/mda5-helicase-domains-identified-as-the-main-targets-of-anti-mda5-autoantibodies-in-european-dermatomyositis-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mda5-helicase-domains-identified-as-the-main-targets-of-anti-mda5-autoantibodies-in-european-dermatomyositis-patients/