Session Information
Date: Monday, November 9, 2020
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster III: Therapies
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Pegloticase is approved for severe gout in patients that are intolerant to, or whose disease is ineffectively controlled by, other uric acid lowering therapies (ULTs). The positioning of pegloticase as the last therapeutic option begs the question, how is gout managed once pegloticase is discontinued? Here we examine treatment following pegloticase for patients in US clinical care.
Methods: The ARN-TRIO Rheumatology registry contains EMR (fielded and open text), lab, procedure, infusion, medical claims, and specialty pharmacy data generated in care of >75,000 patients by ARN, a network of independent practices with >200 rheumatologists across the US. This study included data for gout-diagnosed patients who initiated their last pegloticase course between Jan 2015 and Dec 2019 with >90 days follow-up from pegloticase discontinuation (index). Comparisons were made using t-test for continuous variables and chi-square or Fischer’s exact tests for categorical variables. Time to event analyses were by Kaplan-Meier and log-rank test. To assess consistency of control of sUA < 6 mg/dL, evaluations were limited to patients with 2+ sUA measures within the period of interest.
Results: 70 of 213 pegloticase-treated patients met study criteria; by time of assessment which was ≥90 days past pegloticase discontinuation, 49/70 (70%) initiated a ULT. [FIGURE 1] Median time from index to ULT initiation was 13 days; 76% (37/49) of initiations occurred within 30 days, 90% (44/49) 90 days, and 96% (47/49) 180 days. Median follow up for patients who did not initiate a subsequent ULT was 404 days, greater than but not significantly different from patients that did initiate ULT post-pegloticase (322 days, p=0.270). The absence of kidney disease was significantly associated with ULT initiation (39/47, 83% v. 14/23, 61% non-initiators, p=0.043). Variables NOT associated with ULT initiation within 180 days post-pegloticase included age, gender, race, ethnicity, payer, CVD, diabetes, non-gout rheumatic diseases, duration of prior pegloticase, and serum uric acid (sUA) ≥6 mg/dL during pegloticase or at pegloticase discontinuation. Of the 47 patients that initiated subsequent ULT within 180 days, 22 of 47 had 2+ sUA measures during treatment, and 9/22 (41%) had 2+ sUA ≥6 mg/dL. Two or more sUA results post-pegloticase were provided for 6/23 patients that did not initiate ULT within 180 days; 5/6 (83%) had 2+ sUA ≥6 mg/dL. [FIGURES 2 & 3]
Conclusion: After pegloticase discontinuation, most patients quickly initiated allopurinol. Consistent sUA < 6 ml/dL was not maintained post-pegloticase for half (14/28) of the evaluable patients. As the new 2020 ACR Guidelines for the Management of Gout advises the treat to target approach to maintain sUA < 6 ml/dL, our results highlight the need for new ULTs and/or better strategies to maximize benefit with available therapies.
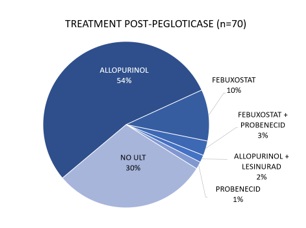 Figure 1: Treatment after pegloticase discontinuation (n=70)
Figure 1: Treatment after pegloticase discontinuation (n=70)
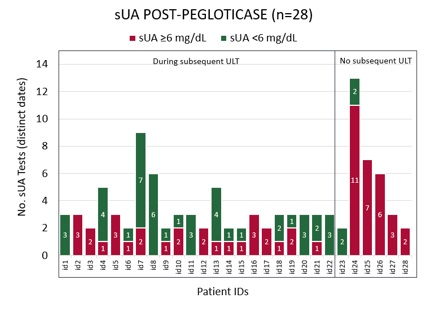 Figure 2: sUA test counts and results during ULT following pegloticase discontinuation (n=28)
Figure 2: sUA test counts and results during ULT following pegloticase discontinuation (n=28)
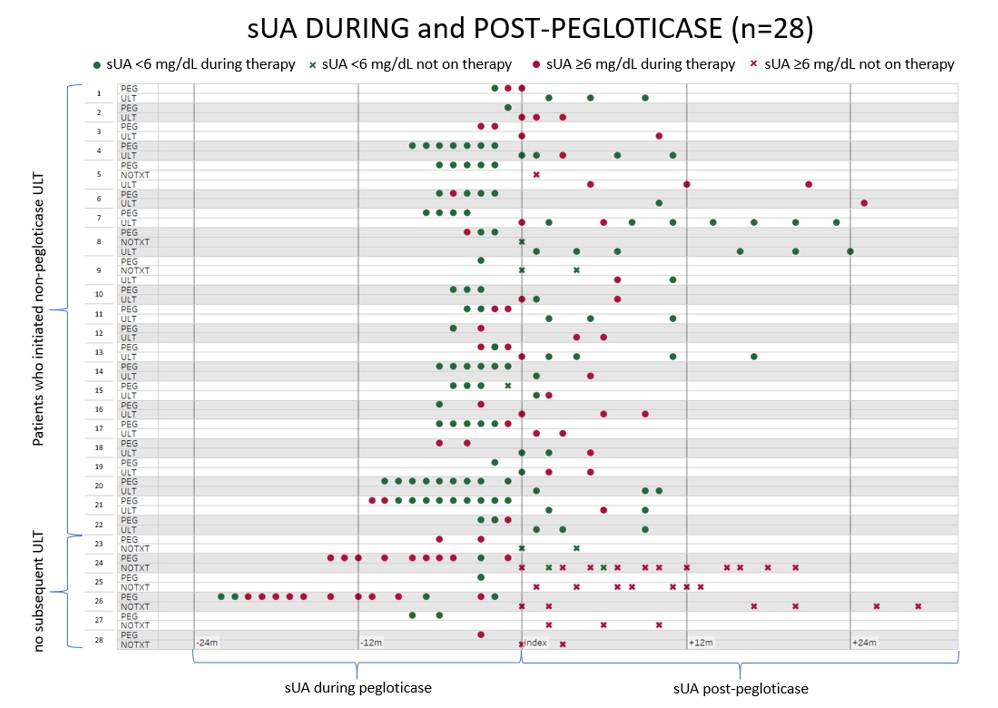 Figure 3: Alternative view of the patients in Figure 2, including sUA results during and after pegloticase treatment (n=28)
Figure 3: Alternative view of the patients in Figure 2, including sUA results during and after pegloticase treatment (n=28)
To cite this abstract in AMA style:
Soloman N, Amin M, Helfgott S, Hu A, Huston K, Leonard J, Milligan K, Milligan S, Singh J, Tesser J, Edgerton C. Management of Gout After Pegloticase; Observations of US Clinical Practice from Trio Health and the American Rheumatology Network (ARN) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/management-of-gout-after-pegloticase-observations-of-us-clinical-practice-from-trio-health-and-the-american-rheumatology-network-arn/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/management-of-gout-after-pegloticase-observations-of-us-clinical-practice-from-trio-health-and-the-american-rheumatology-network-arn/
