Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Malondialdehyde and acetaldehyde, by-products of lipid peroxidation, react with free amino groups on proteins to form a stable post-translational modification (PTM), termed MAA. MAA modifications and associated anti-MAA antibodies have been implicated in the pathogenesis of rheumatoid arthritis (RA). In RA synovium, MAA adducts have been shown to co-localize with another PTM, citrullination. Citrullination is mediated by the calcium-dependent enzyme peptidyl arginine deiminase (PAD), and its activity is dependent on tightly regulated intracellular calcium concentrations. One such intracellular calcium regulator is matrix gla protein (MGP), which functions as an intracellular calcium scavenger. The goal of this study was to evaluate potential changes in PAD expression and citrullination that may occur as a result of MAA modification of MGP.
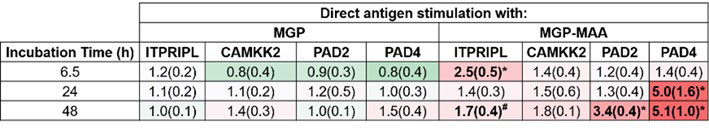
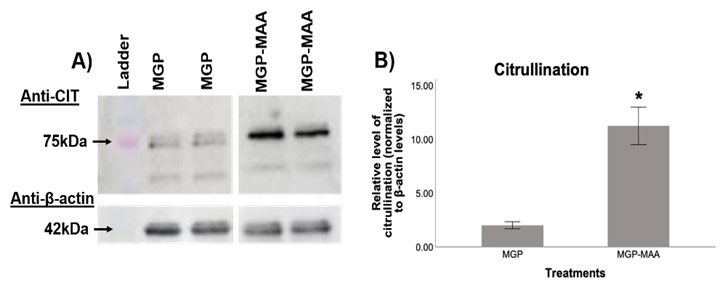
Methods: A human monocytic cell line (U-937) was differentiated to a macrophage phenotype using phorbol 12-myristate 13-acetate (PMA), and subsequently stimulated for 6.5, 24, and 48 hours with either; native MGP or MAA-modified MGP (MGP-MAA). RT-PCR was performed for the following calcium modulators: inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4. Additionally, antigen-stimulated U-937 cells were lysed for protein purification and analyzed using Western Blotting for citrullinated (CIT) proteins using an anti-CIT antibody. The relative expression of protein quantity was normalized to β-actin (endogenous control).
Results: In comparison to native MGP, differentiated U-937 cells stimulated with MGP-MAA demonstrated an increase in mRNA expression for ITPRIPL (2.5-fold, p< 0.001) at 6.5 hours, PAD2 (3.4-fold, p< 0.001) at 48 hours, and PAD4 (5-fold, p< 0.001) at both 24 and 48h (Table 1). For Western Blot, MGP-MAA stimulated cells showed a 10-fold increase (p< 0.001) in citrullinated proteins compared to native MGP stimulated cells with the most prominent band identified at 75 kDA, which is suspected to be PAD2 based on preliminary data (Figure 1).
Conclusion: Although mechanisms underpinning these observations need to be elucidated, these studies suggest that MAA adduction of MGP likely diminishes its function as a calcium scavenger, resulting in an upregulation of key cellular calcium binding proteins. Amongst them is the calcium-dependent enzyme PAD2 and PAD4, which catalyzes the citrullination of proteins. The identification of a prominent 75 kDA band (a molecular weight corresponding to PAD) suggests the possibility that stimulation of cells with MAA-modified MGP may lead to the auto-citrullination of PAD. Taken together, these findings provide insight into a potential mechanism by which increased levels of citrullinated proteins in macrophages are produced resulting in the formation of autoantigens and autoantibodies unique to RA.
To cite this abstract in AMA style:
Jones S, Aripova n, Duryee M, Ragland A, England B, Mikuls T, Thiele G. Malondialdehyde-Acetaldehyde (MAA) Modified Matrix Gla Protein (MGP) Increases Citrullination by Human Macrophages [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/malondialdehyde-acetaldehyde-maa-modified-matrix-gla-protein-mgp-increases-citrullination-by-human-macrophages/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/malondialdehyde-acetaldehyde-maa-modified-matrix-gla-protein-mgp-increases-citrullination-by-human-macrophages/