Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Individuals with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared to the general population, which is partly explained by systemic inflammation. While emerging evidence suggests tumor necrosis factor inhibitors (TNFi) may be cardioprotective, there are safety concerns for Janus kinase inhibitors (JAKibs) in regard to major adverse cardiovascular events (MACE) and venous thromboembolism (VTE). We aimed to assess the risk of MACE and VTE in axSpA and PsA comparing users of JAKibs versus TNFi.
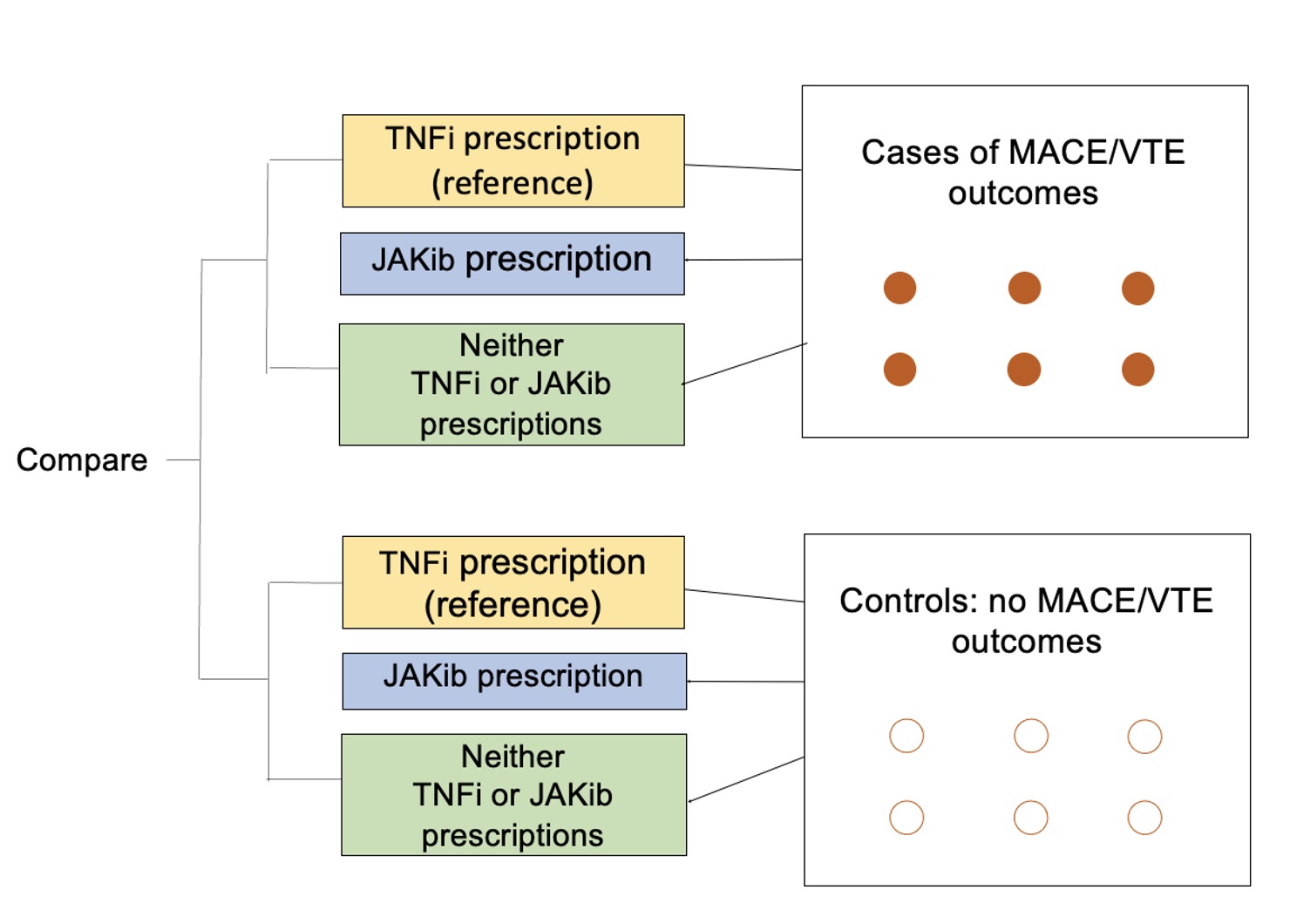
Methods: We conducted two nested case-control studies (Figure 1) using 2006-2021 data from the MarketScan Commercial Claims and Encounters (Merative) Database, which includes de-identified, patient-specific data of reimbursed healthcare claims. We included adults aged 18-65 years with ≥1 inpatient or 2 outpatient axSpA or PsA ICD-9/-10 diagnosis codes separated by ≥7 days.
For the first case control study, MACE was defined as the first inpatient claim for myocardial infarction or stroke/transient ischemic attack; for the second case control study, VTE was defined using the first inpatient/outpatient claim. The index date was the date of the MACE or VTE outcome among cases; or matching the case’s outcome date among controls. Cases and controls were matched 1:4 by age, gender and axSpA/PsA diagnosis date using risk-set sampling. We evaluated drug exposure (TNFi or JAKib use or neither) using pharmacy claims and CPT codes within 6 months prior to the index date.
We assessed the association of drug exposure (referent: TNFi use) with the outcomes of MACE and VTE in separate models, using conditional logistic regression with adjustment for potential confounders (age, cardiovascular disease history, comorbidities, and tobacco use) to calculate odds ratios (OR) and 95% confidence intervals (CI).
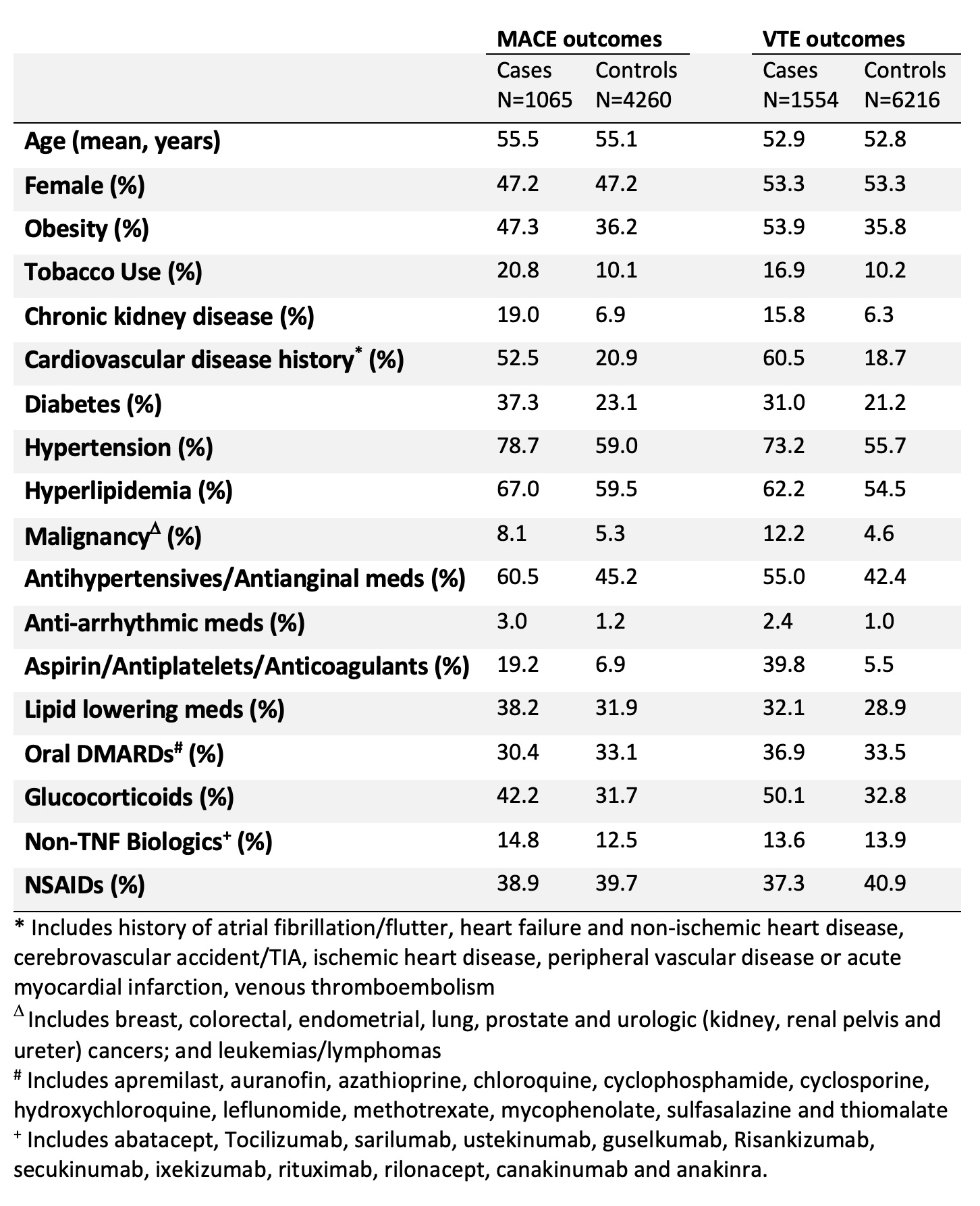
Results: We identified 1065 MACE cases among 5325 adults with AxSpA or PSA and 1554 VTE cases among 7770 axSpA/PsA subjects. Demographics and clinical characteristics are shown in Table 1. Comorbidities including cardiovascular disease history, obesity, diabetes and hypertension were more commonly seen in cases versus controls.
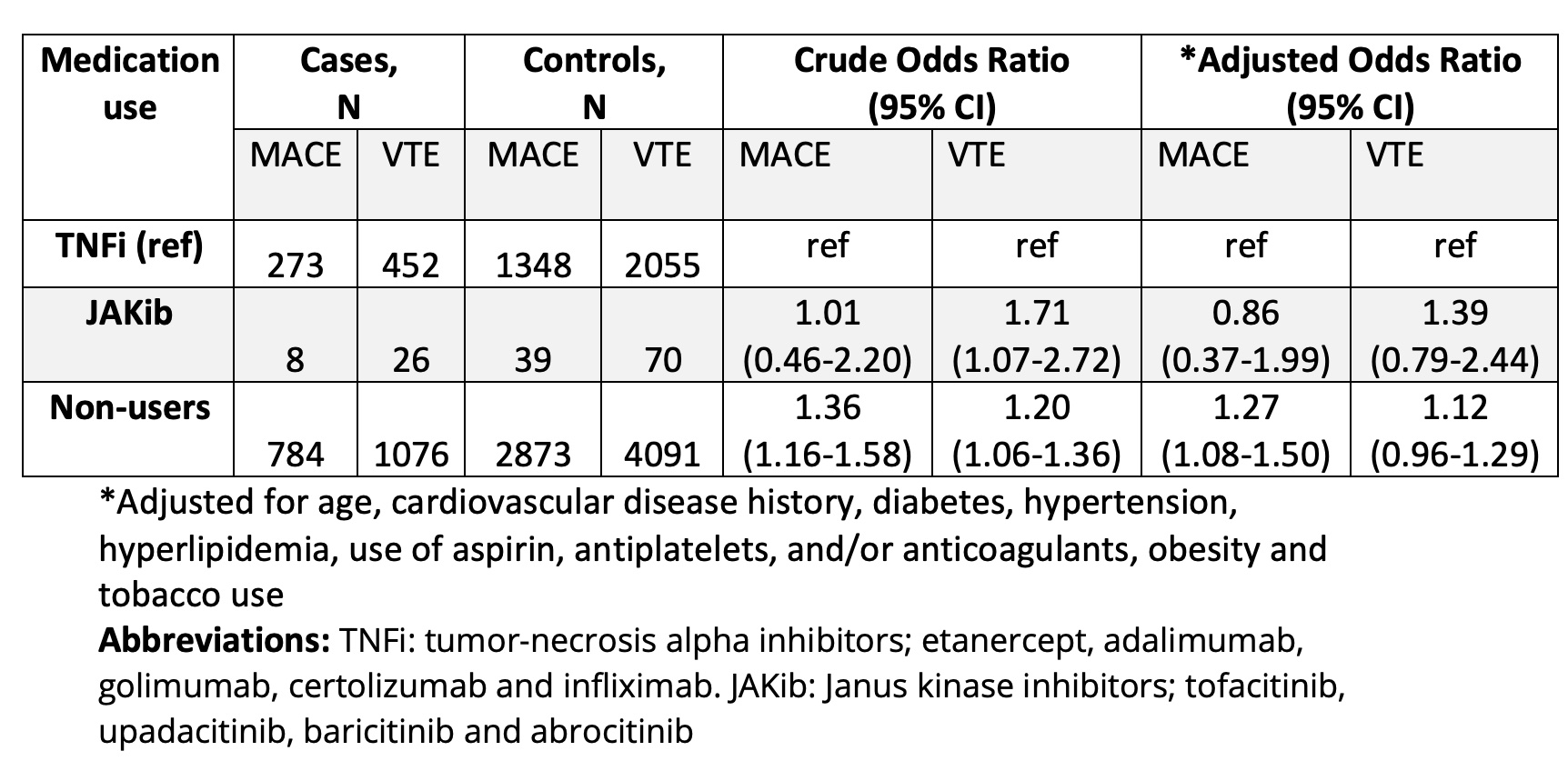
In the MACE nested case control study, 47 (0.9%) were JAKib users, 1621 (30.4%) were TNFi users and 3657 (68.7%) were non-users of JAKib and TNFi. In the VTE sample, 96 (1.2%) were JAKib users, 2507 (32.3%) were TNFi users and 5167 (66.5%) were non-users.
Non-users of TNFi and JAKibs had 1.3 times the odds of MACE relative to TNFi users (95% CI 1.08-1.50; Table 2). In JAKib users versus TNFi users, the odds of MACE was not increased (OR 0.86, 95% CI 0.37-1.99). The odds ratio for VTE was 1.39 among JAKib users however, this was not statistically significant after confounder adjustment (95% CI 0.79-2.44).
Conclusion: Using a large US insurance claims database, we did not find a statistically significant association between JAKib use and risk of MACE or VTE, compared with TNFi use among individuals with axSpA and PsA, although there was a trend toward increased VTE events among JAKib users. The accrual of further data is needed to better study the comparative safety of JAKibs versus TNFi in this study population.
To cite this abstract in AMA style:
Merjanah S, Driscoll D, Peloquin C, liew J, Dubreuil M. Major Adverse Cardiovascular Event and Venous Thromboembolism Risk Comparing Advanced Therapies Among Individuals with Axial Spondylarthritis and Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/major-adverse-cardiovascular-event-and-venous-thromboembolism-risk-comparing-advanced-therapies-among-individuals-with-axial-spondylarthritis-and-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/major-adverse-cardiovascular-event-and-venous-thromboembolism-risk-comparing-advanced-therapies-among-individuals-with-axial-spondylarthritis-and-psoriatic-arthritis/