Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The analysis of the EUSTAR Very Early Diagnosis of Systemic Sclerosis (VEDOSS) study has identified the risk of progression to fulfil 2013 ACR/EULAR classification criteria in patients with Raynaud’s and one or more “red flag” signs, which include Antinuclear antibodies (ANA), Nailfold capillaroscopy (NVC) changes, and puffy fingers. In this sense, the analysis supported a very early diagnosis in patients not fulfilling classification criteria if they were presenting with at least one red flag sign. Here we aimed to implement an unbiased machine learning approach to identify and rank other clinical and demographic features collected within the VEDOSS EUSTAR database that would predict progression to fulfil classification criteria for SSc.
Methods: The VEDOSS study was designed as a non-interventional, prospective observational cohort of the EUSTAR registry, as described elsewhere (Bellando-Randone et al, 2021). Database download was performed on date 08/04/2023. The outcome was an event-based endpoint of progression to fulfil 2013 ACR/EULAR classification criteria for SSc. Demographic and clinical predictor variables for progression were scored for their relevance, using tree-based machine learning approaches, namely extreme gradient (XG) boosting and random forest. New covariates emerging significant in both tree-based iterations were modelled on the whole cohort using Kaplan-Meier (KM) survival curves.
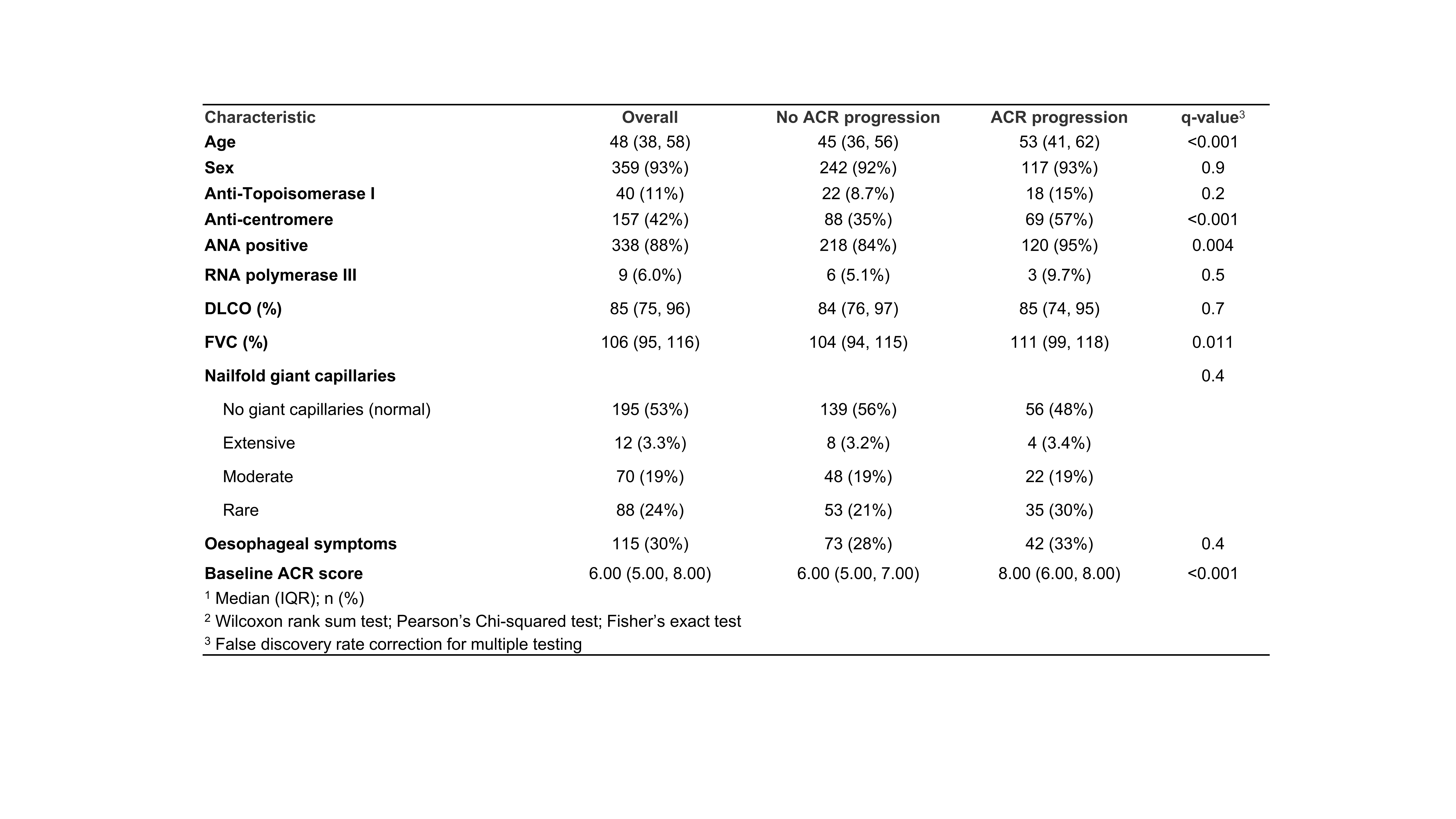
Results: 601 patients not scoring 9 ACR/EULAR criteria points at baseline with at least two visits were available for the analysis, demographic and clinical characteristics are presented in table 1. XG boosting analysis identified the following as the five most important variables for gain in predictive ability at 5-year: oesophageal symptoms (gain 0.173), ACA (gain 0.156), ATA (gain 0.147), giant capillaries (0.124), and puffy fingers (gain 0.096). Random Forest identified giant capillaries (6.986), ATA (5.983), oesophageal symptoms (5.642), bushy capillaries (5.065), and ACA (4.977) as the most important variables.
Of 229 cases without censoring and at least 5-year follow-up, positive for both baseline RP and ANA positivity, 47% showed ACR/EULAR criteria progression at 5 years. Focusing on a subset of 122/229 with RP, ANA, and oesophageal symptoms as new identified red flag, these showed a progression rate of 55%, with an improvement of 8%. KM curves of all 601 patients stratified by ANA, SSc-specific antibodies, and oesophageal symptoms, showed that the addition of oesophageal symptoms to SSc-specific antibodies identified patients with a significantly shorter time-to-progression.
Conclusion: Oesophageal symptoms were found to improve the accuracy of the established VEDOSS criteria for identification of progressors. This analysis of the largest multicentre cohort for very early SSc supports the rationale of considering oesophageal symptoms in the context of Raynaud’s and ANA as a new red flag for early detection of SSc.
To cite this abstract in AMA style:
Di Donato S, Kakkar V, Bellando-Randone S, Matucci-Cerinic M, Del Galdo F. Machine Learning Identifies Oesophageal Symptoms as a New Red Flag for for Very Early Diagnosis of Systemic Sclerosis (VEDOSS): A EUSTAR Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/machine-learning-identifies-oesophageal-symptoms-as-a-new-red-flag-for-for-very-early-diagnosis-of-systemic-sclerosis-vedoss-a-eustar-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/machine-learning-identifies-oesophageal-symptoms-as-a-new-red-flag-for-for-very-early-diagnosis-of-systemic-sclerosis-vedoss-a-eustar-analysis/