Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: In rheumatoid arthritis (RA) synovium, activated synovial fibroblasts and macrophages release inflammatory mediators that affect surrounding cells and accelerate disease progression. One such chemokine axis, we and others have shown to be present in RA, is CX3CL1/Fractalkine ligand and its receptor CX3CR1(Fractalkine receptor). Malondialdehyde and acetaldehyde adducts (MAA) and/or citrullinated (CIT) proteins in isolation or in combination induce inflammatory and fibrotic responses by macrophages and fibroblasts. Recent preliminary data from our laboratory using the VARA cohort (N=2342) suggests that anti-MAA antibodies negatively correlated with serum CX3CL1 levels (R=-0.04123, p< 0.05). Therefore, it was the purpose of this study to evaluate the effects of MAA and/or CIT modified proteins on the expression of CX3CL1 and CX3CR1 by macrophages (U937 cells), and human fibroblast-like synoviocytes from RA synovium (HFLS-RA).
Methods: Both HFLS-RA and U937 cells, separately, were stimulated with MAA, CIT, and MAA-CIT modified or unmodified human serum albumin (HSA) or fibrinogen (FIB) for 24 hours. Afterward, supernatants were assayed using a cytokine capture assay for CX3CL1. CX3CR1 expression was measured using both PCR and flow cytometry. In a separate experiment, U937 cells were incubated with previously collected HFLS-RA supernatants (following antigen stimulation as above) and mRNA was isolated to evaluate inflammatory markers with PCR: TNF-α, IL-1β. One-way ANOVA with Tukey’s-b post-hoc was used to compare normalized values across exposure groups to appropriate native proteins.
Results: U937 cells do not secrete the CX3CL1, and HFLS-RA cells do not express the CX3CR1 in response to any modification of HSA or FIB. However, stimulation of U937 with modified antigens significantly increased CX3CR1 expression by flow cytometry [Fig.1A,B]. Stimulation with dually modified (MAA-CIT) HSA and FIB demonstrated the highest CX3CR1 expression by PCR [Fig.1C]. Treatment of HFLS-RA with HSA-MAA had the highest significant CX3CL1 release, followed by HSA-MAA-CIT [Fig.2A]. Treatment with FIB-MAA-CIT significantly increased levels of CX3CL1 release, followed by FIB-CIT [Fig.2B]. When U937 cells were stimulated with HSA-MAA-CIT [Fig.3A] or FIB-CIT [Fig.3B] supernatants from HFLS-RA cells, mRNA levels of TNF-α and IL-1β increased, but to a significantly lesser extent than respective stimulations without HFLS-RA supernatants. HSA-MAA treated HFLS-RA supernatants significantly increased U937 mRNA levels for TNF-α and considerably, to a lesser extent, IL-1β as compared to direct stimulation of U937 with HSA-MAA antigen without HFLS-RA supernatants [Fig.3A].
Conclusion: Our data demonstrate for the first time that fibroblasts and macrophages can express CX3CL1 and CX3CR1, respectively, in response to HSA and FIB modified proteins. Macrophages showed significantly diminished inflammatory responses when exposed to supernatants from stimulated HFLS-RA cells. These studies suggest that the CX3CL1-CX3CR1 axis could link immune cells and fibroblasts, thereby regulating inflammatory mediators in response to MAA and/or CIT modified proteins.
 Figure 1. Altered CX3CR1/Fractalkine receptor expression from monocytes stimulated with post-translationally modified albumin and fibrinogen. U937 cells were incubated with human serum albumin (HSA) and fibrinogen (FIB) modified antigens. Panels (A) and (B) show CX3CR1/Fractalkine receptor expression from flow cytometry with HSA (A) and FIB (B) modified antigens; the data are represented using mean fluorescent intensity (MFI) and are normalized tβ o native protein. Panel (C) summarizes mean relative quantity (Rq) mRNA levels of CX3CR1/Fractalkine receptor by PCR. *p < 0.001, n=3.
Figure 1. Altered CX3CR1/Fractalkine receptor expression from monocytes stimulated with post-translationally modified albumin and fibrinogen. U937 cells were incubated with human serum albumin (HSA) and fibrinogen (FIB) modified antigens. Panels (A) and (B) show CX3CR1/Fractalkine receptor expression from flow cytometry with HSA (A) and FIB (B) modified antigens; the data are represented using mean fluorescent intensity (MFI) and are normalized tβ o native protein. Panel (C) summarizes mean relative quantity (Rq) mRNA levels of CX3CR1/Fractalkine receptor by PCR. *p < 0.001, n=3.
 Figure 2. CX3CL1/Fractalkine ligand release from stimulated HFLS-RA cells. HFLS-RA cells were incubated with HSA (A) and FIB (B) modified antigens for 24 hours. Afterwards, the supernatants were collected for analysis for CX3CL1/Fractalkine ligand release using Mesoscale Discovery assay kit. The concentration of CX3CL1/Fractalkine ligand was measured in pg/mL and normalized to native protein. Log based 10 ratio of modified protein to native protein is represented on y-axis (HSA and FIB respectively). *p < 0.001, n=3.
Figure 2. CX3CL1/Fractalkine ligand release from stimulated HFLS-RA cells. HFLS-RA cells were incubated with HSA (A) and FIB (B) modified antigens for 24 hours. Afterwards, the supernatants were collected for analysis for CX3CL1/Fractalkine ligand release using Mesoscale Discovery assay kit. The concentration of CX3CL1/Fractalkine ligand was measured in pg/mL and normalized to native protein. Log based 10 ratio of modified protein to native protein is represented on y-axis (HSA and FIB respectively). *p < 0.001, n=3.
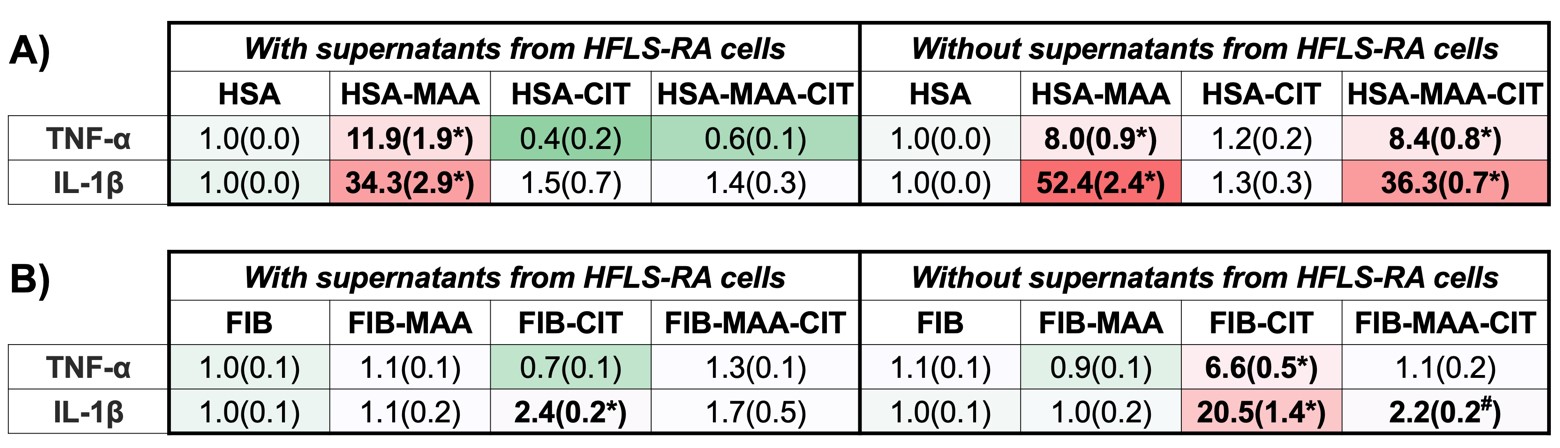 Figure 3. PCR for mRNA levels of inflammatory markers from stimulated U937 cells. U937 cells were stimulated with either supernatants from modified antigens treated HFLS-RA cells or with directly modified antigens. RNA was collected from U937 cells and categorized as incubation with HSA (A) or FIB (B) modified antigens. The data is represented as relative quantity (Rq) of inflammatory markers. #p < 0.05, *p < 0.001, n=3.
Figure 3. PCR for mRNA levels of inflammatory markers from stimulated U937 cells. U937 cells were stimulated with either supernatants from modified antigens treated HFLS-RA cells or with directly modified antigens. RNA was collected from U937 cells and categorized as incubation with HSA (A) or FIB (B) modified antigens. The data is represented as relative quantity (Rq) of inflammatory markers. #p < 0.05, *p < 0.001, n=3.
To cite this abstract in AMA style:
Aripova N, Duryee M, Maloley P, England B, O'Dell J, Mikuls T, Thiele G. MAA Modified and/or Citrullinated Proteins Stimulate Macrophages and Human Fibroblast-Like Synoviocytes to Increase the Secretion/Expression of Fractalkine Ligand (CX3CL1) and Fractalkine Receptor (CX3CR1) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/maa-modified-and-or-citrullinated-proteins-stimulate-macrophages-and-human-fibroblast-like-synoviocytes-to-increase-the-secretion-expression-of-fractalkine-ligand-cx3cl1-and-fractalkine-receptor-cx/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maa-modified-and-or-citrullinated-proteins-stimulate-macrophages-and-human-fibroblast-like-synoviocytes-to-increase-the-secretion-expression-of-fractalkine-ligand-cx3cl1-and-fractalkine-receptor-cx/
