Session Information
Date: Monday, November 8, 2021
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: The alarmin interleukin (IL)-33 is a member of the IL-1 cytokine family that is rapidly released from the nucleus of a variety of lung cells upon cellular damage or xenobiotic (including airborne biohazard) exposures. IL-33 has been implicated in rheumatoid arthritis (RA), where increased levels in sera and synovial fluid correlate with disease activity. Moreover, elevated sera and lung IL-33 occur in idiopathic pulmonary fibrosis, a condition that resembles RA-interstitial lung disease. However, IL-33 levels and its role in the pathogenesis of RA-associated lung disease are not known.
Methods: Formalin-fixed, paraffin-embedded (FFPE) de-identified human lung sections from patients with RA-lung disease (N=9) and normal “control” (N=7) donors (deemed unsuitable for transplant) were obtained from the local institutional IRB-approved biobanks. An animal model combining the collagen-induced arthritis (CIA) and 5 weeks of repetitive inhalation of lipopolysaccharide (LPS, 100 ng) was utilized to obtain lung sections from 4 groups of DBA/1J mice (N=5/group): 1) Sham (saline injection/saline inhalations); 2) CIA (CIA injections/saline inhalations); 3) LPS (saline injections/LPS inhalations); and 4) CIA+LPS. Sections were stained for human or mouse anti-IL-33 and vimentin. Photographs (10 per lung section per patient/mouse) were taken using a Zeiss fluorescent microscope. Integrated densities (the product of area and mean gray value) of each protein were measured as a single color on black background with color thresholds determined by Image J.
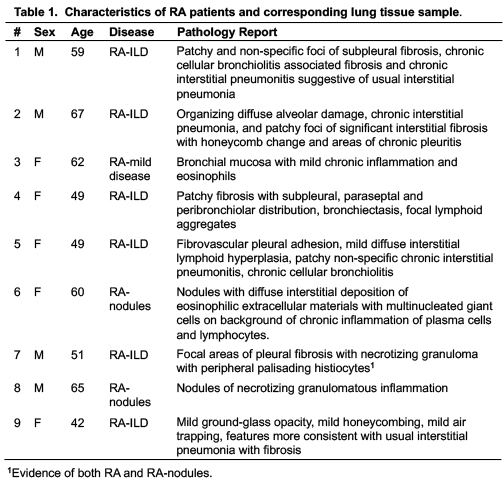
Results: Characteristics of patients with RA-associated lung disease and corresponding lung tissue pathology are summarized in Table 1. Lung IL-33 expression was strikingly increased in the lung tissues of patients with RA-associated lung disease (5.7-fold), with lesser, but significant, increases in vimentin staining (1.9-fold) (p=0.0002 and p=0.0033, respectively) relative to control lung tissues (Figure 1). In sensitivity analyses excluding 2 subjects with RA-related pulmonary nodules and 1 subject with mild inflammation, IL-33 and vimentin expression remained significantly elevated (p < 0.01 for both). In murine modeling studies, lung IL-33 expression was increased with LPS alone (5.3-fold) and CIA+LPS (5.7-fold) vs. Sham (p < 0.001) and vs. CIA alone (3.1-fold and 3.4-fold, respectively, p< 0.001) with no significant difference between LPS and CIA+LPS (Figure 2). Vimentin expression was increased in CIA (2.6-fold, p< 0.01), LPS (2.8-fold, p< 0.05) and CIA+LPS (3.8-fold, p< 0.01) vs. Sham.
Conclusion: This study demonstrates that lung IL-33 expression is increased in patients with RA-associated lung disease. In the combined inhalant LPS+CIA animal modeling studies, lung IL-33 was also elevated, primarily driven by LPS exposure. As clinical studies are underway utilizing anti-IL-33/ST2 pathway antibodies in COPD, this approach could be explored in RA-associated lung disease. Alternatively, IL-33 warrants further investigation as a biomarker to identify patients with RA who are at risk of developing RA-associated lung disease.
 Figure 1. Lung IL_33 and vimentin expression in human tissue sections. Photomicrographs were taken of entire lung section using a Zeiss fluorescent microscope. (A) A representative image of individual and merged stains of IL_33 (red), vimentin (green) and DAPI (blue; nuclei), with a zoomed image of an area from a control subject and patient with RA-LD (RA-associated lung disease). Scatter plots with bar graph for IL_33 (B) and vimentin (C) with p values. Red dots denote females and blue dots denote males in each group.
Figure 1. Lung IL_33 and vimentin expression in human tissue sections. Photomicrographs were taken of entire lung section using a Zeiss fluorescent microscope. (A) A representative image of individual and merged stains of IL_33 (red), vimentin (green) and DAPI (blue; nuclei), with a zoomed image of an area from a control subject and patient with RA-LD (RA-associated lung disease). Scatter plots with bar graph for IL_33 (B) and vimentin (C) with p values. Red dots denote females and blue dots denote males in each group.
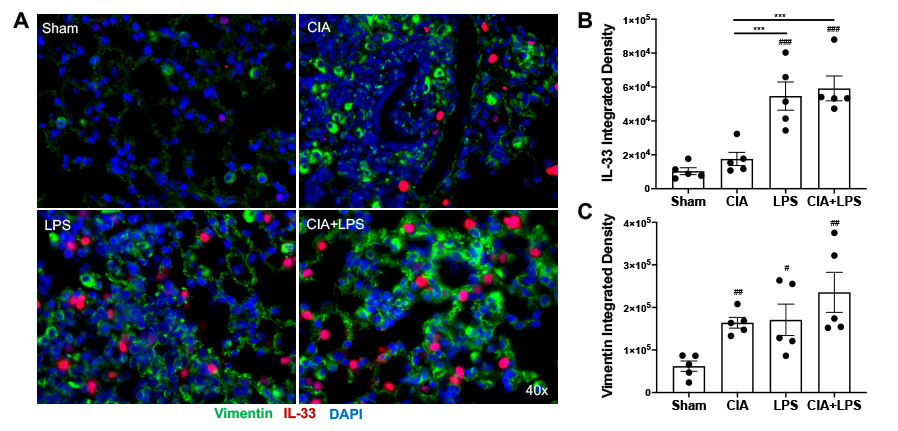 Figure 2. Combined modeling of repetitive lipopolysaccharide (LPS)-induced airway inflammation and collagen-induced arthritis (CIA) demonstrates increases in lung IL_33 expression. Photomicrographs were taken of entire lung section using a Zeiss fluorescent microscope. (A) A representative merged image of all three stains of IL_33 (red), vimentin (green) and DAPI (blue; nuclei) from each murine treatment group. Nf5 mice/group. Scatter plots with bar graph for IL_33 (B) and vimentin (C). Statistical differences are shown for treatment groups versus sham (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001) and between treatment groups (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Figure 2. Combined modeling of repetitive lipopolysaccharide (LPS)-induced airway inflammation and collagen-induced arthritis (CIA) demonstrates increases in lung IL_33 expression. Photomicrographs were taken of entire lung section using a Zeiss fluorescent microscope. (A) A representative merged image of all three stains of IL_33 (red), vimentin (green) and DAPI (blue; nuclei) from each murine treatment group. Nf5 mice/group. Scatter plots with bar graph for IL_33 (B) and vimentin (C). Statistical differences are shown for treatment groups versus sham (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001) and between treatment groups (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
To cite this abstract in AMA style:
Gaurav R, Mikuls T, Thiele G, England B, Wolfe M, Bailey K, Nelson A, Duryee M, Romberger D, Ascherman D, Poole J. Lung Interleukin-33 Is Elevated in Rheumatoid Arthritis-Associated Lung Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/lung-interleukin-33-is-elevated-in-rheumatoid-arthritis-associated-lung-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lung-interleukin-33-is-elevated-in-rheumatoid-arthritis-associated-lung-disease/