Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Rituximab impairs humoral immunity by depleting CD20+ B cells. Previous studies have demonstrated that patients on rituximab have decreased immune response to COVID-19 vaccination. However, prior studies have not fully delineated the effect of patient characteristics, treatment protocol, timing of vaccination, and B cell levels on antibody response. This study aims to assess how patient characteristics impact COVID-19 seroconversion in patients treated with rituximab.
Methods: This was a single-center retrospective cohort study including 77 patients with rheumatoid arthritis (RA), ANCA-associated vasculitis (AAV), and SLE/mixed connective tissue disease who obtained COVID-19 antibody testing at clinic visits from May to December 2021. 48 (62%) of these patients received rituximab. The Roche Elecsys anti-SARS-CoV-2 (S) immunoassay was used to determine the presence of antibodies. Seroconversion was defined by antibodies ≥ 0.8 U/mL. Characteristics of rituximab-receiving patients that seroconverted were compared to those that did not seroconvert, using Fisher’s exact test for categorical variables and a student’s t-test for continuous variables. A multivariable logistic regression was used to adjust for selected covariates.
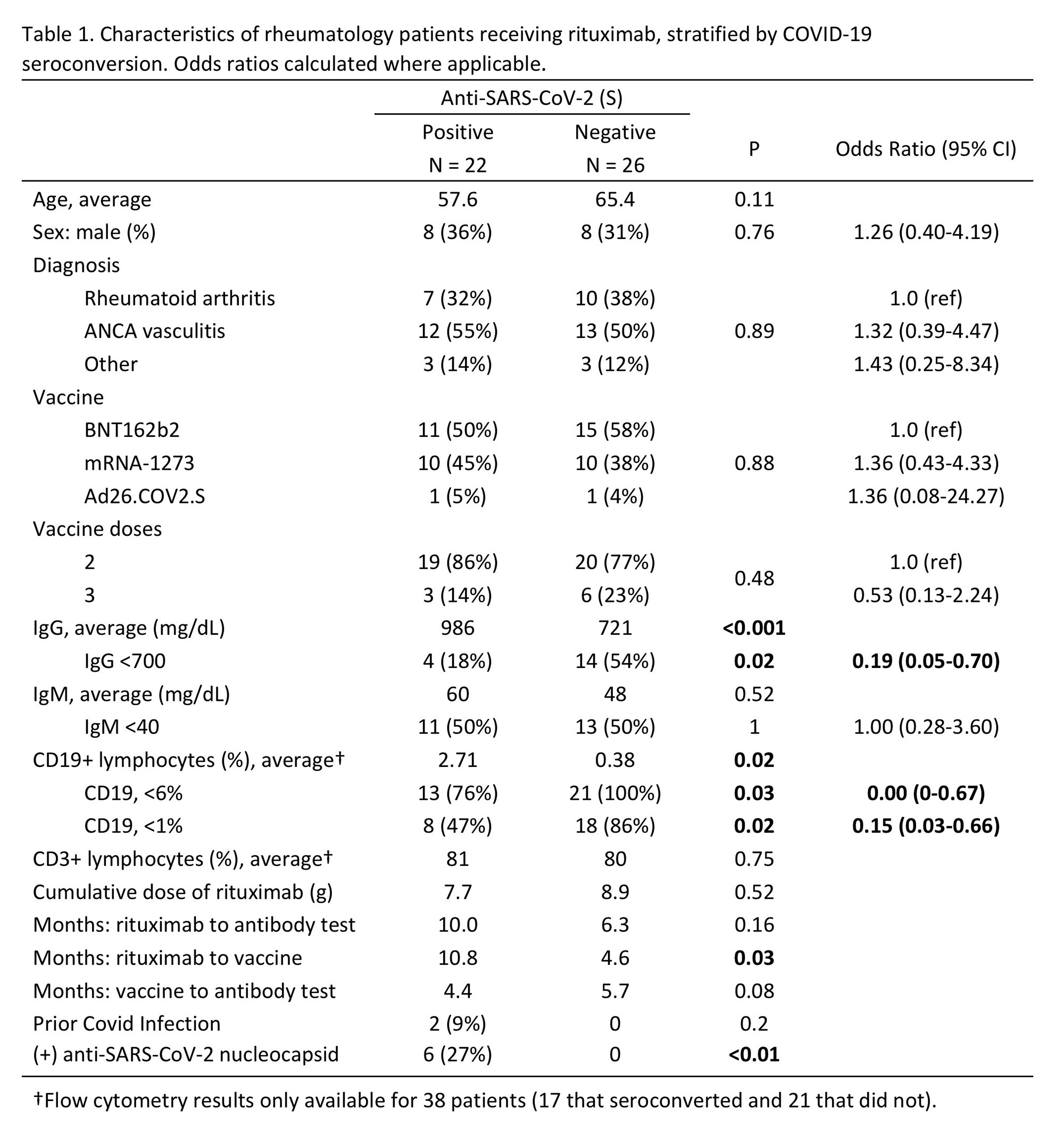
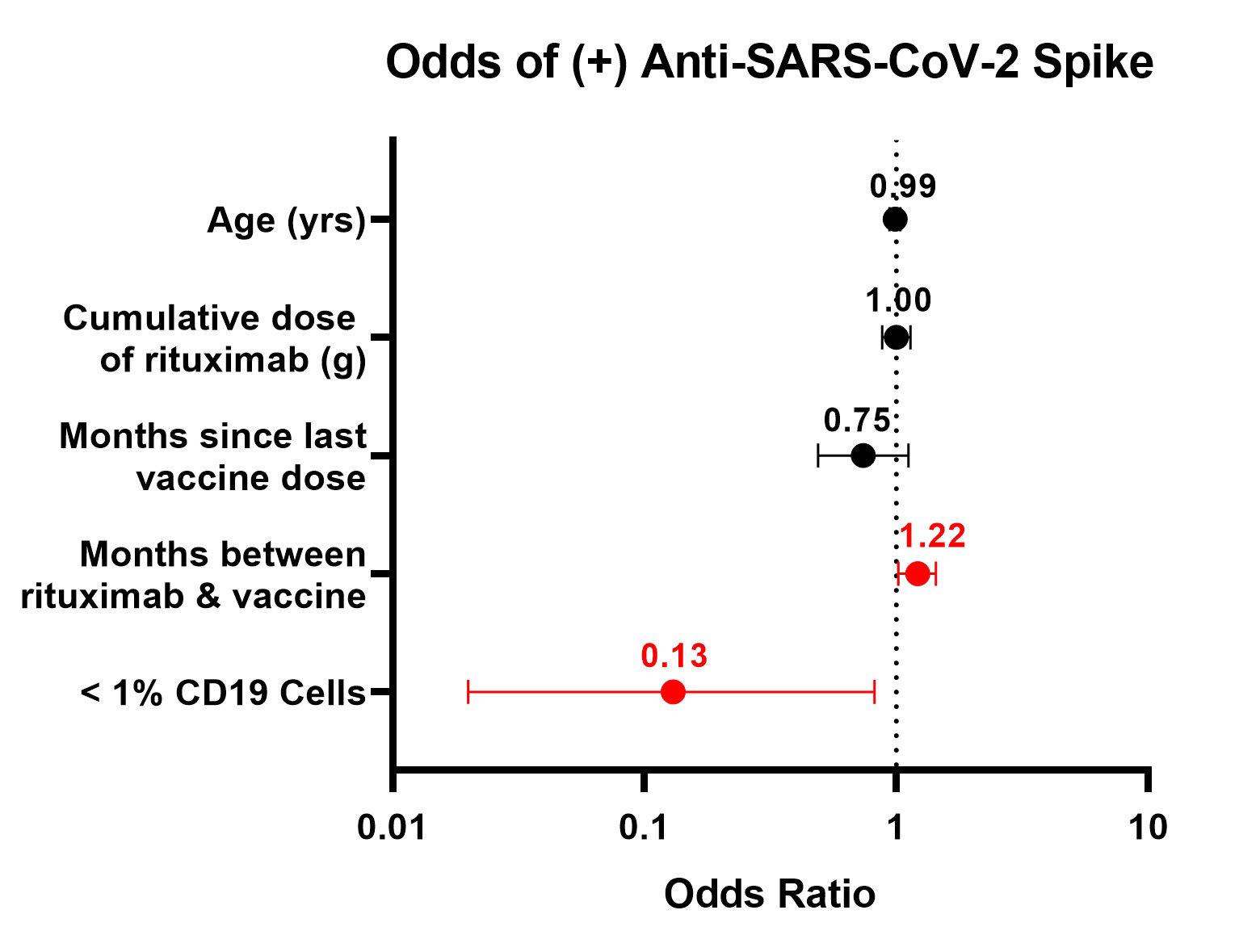
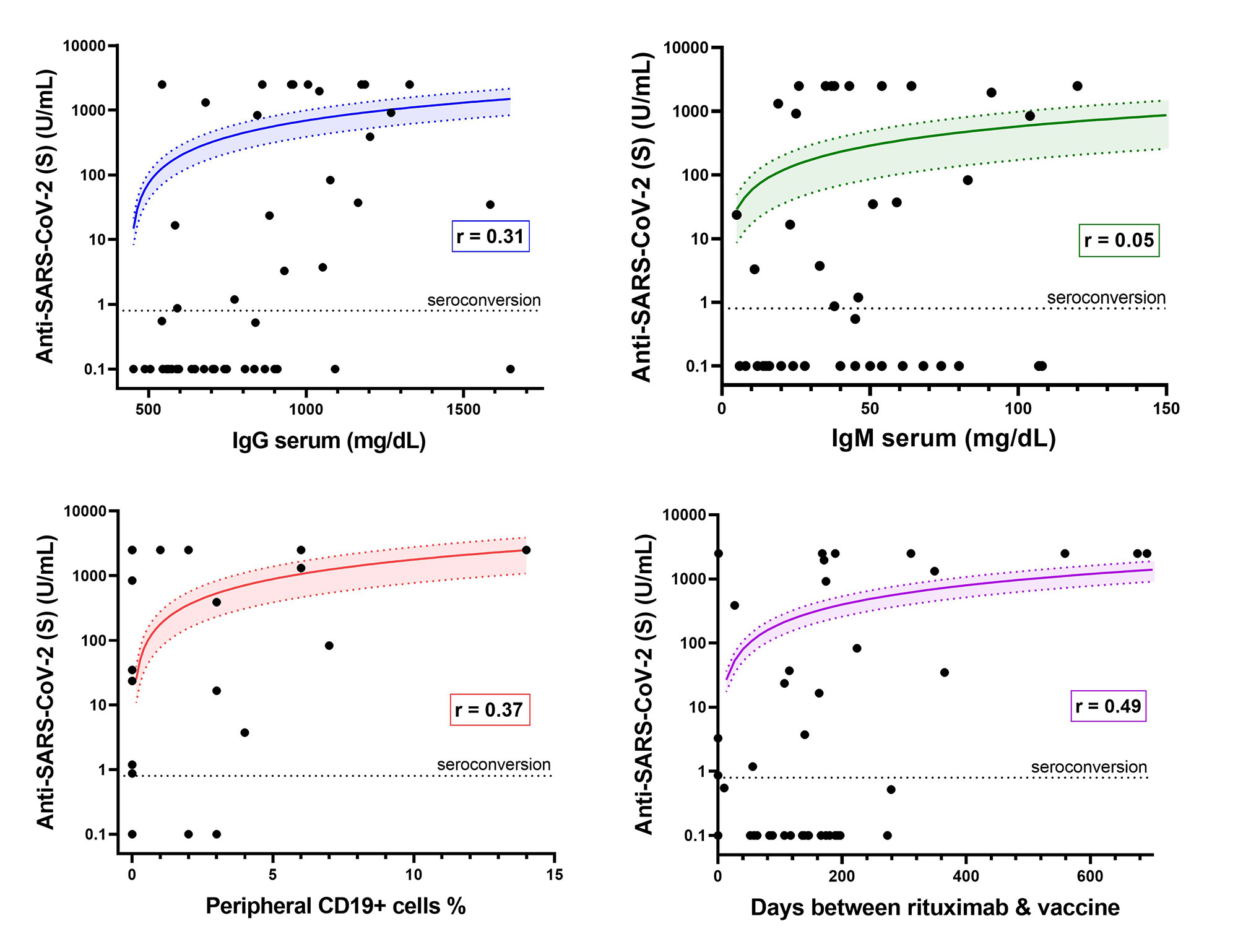
Results: Fewer patients on rituximab seroconverted (46%) compared to those treated with non-B cell targeting agents (86%) (p< 0.001). Among patients on rituximab, factors associated with seroconversion included longer duration between rituximab and 2nd vaccine (p=0.03), presence of anti-nucleocapsid antibodies (p< 0.01), more CD19+ lymphocytes (p=0.02), and greater serum IgG levels (p< 0.001) (Table 1). Patients with serum IgG ≤700 mg/dL had lower odds of seroconversion than patients with IgG >700 mg/dL (OR=0.19; 95% CI: 0.05-0.70). Patients with < 1% CD19+ lymphocytes were less likely to have anti-S antibodies than patients with higher B cell levels (OR=0.15; CI: 0.03-0.66). < 1% CD19 remained associated with lower odds of seroconversion after adjustment for covariates (aOR=0.13; 95% CI: 0.02-0.82) (Figure 1). Quantitative anti-S titer was positively correlated with serum IgG (r=0.31), %CD19+ lymphocytes (r=0.37), and time between rituximab and vaccine (r=0.49) (Figure 2).
There was no difference in seroconversion between patients with AAV and RA (p=0.76). There was no difference in seroconversion based on patient age, sex, vaccine received, number of vaccine doses, months between vaccine and antibody test, or %CD3+ lymphocytes. Six patients (13%) developed subsequent COVID-19 infection as of May 2022; none required hospitalization. Eight patients that did not initially have antibodies were re-tested after receiving a booster vaccine within 6 months. Only one of these patients seroconverted following a booster dose.
Conclusion: Despite different treatment protocols, patients with rheumatoid arthritis and ANCA-associated vasculitis on rituximab had similar rates of antibody response to COVID-19 vaccination. Low levels of circulating B cells and serum IgG levels were associated with decreased antibody response. This study adds to a growing body of evidence that treatment with rituximab is associated with suppressed response to COVID-19 vaccination.
To cite this abstract in AMA style:
Halbur C, Sternhagen E, Dimachkie M, Lenert P. Low Circulating B Cells Correlate with Unresponsiveness to COVID-19 Vaccination Among Patients Treated with Rituximab [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/low-circulating-b-cells-correlate-with-unresponsiveness-to-covid-19-vaccination-among-patients-treated-with-rituximab/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-circulating-b-cells-correlate-with-unresponsiveness-to-covid-19-vaccination-among-patients-treated-with-rituximab/