Session Information
Session Type: Abstract Session
Session Time: 4:00PM-4:15PM
Background/Purpose: Juvenile systemic sclerosis (jSSc) is a rare, life-threatening autoimmune disease characterized by fibrosis and immune dysregulation. Autologous stem cell transplant (ASCT) is an emerging treatment aimed at resetting immune homeostasis. Here, we present the first integrated longitudinal single-cell analysis of paired peripheral blood and skin samples in jSSc patients undergoing ASCT, using Cellular Indexing of Transcriptomes and Epitopes by sequencing (CITE-seq) in context with skin single-cell RNA sequencing (scRNA seq) data.
Methods: Peripheral blood mononuclear cell (PBMC) and skin samples were collected from three jSSc patients pre-ASCT and at 6, 12, and 24 months post-ASCT. Samples were multiplexed per patient including the different timepoints. PBMC and skin samples underwent CITE-seq and scRNA-seq respectively. Data were processed using CellRanger, Seurat, SoupX, DSB, Harmony, and RPCA. PBMCs were clustered using a shared RNA and protein weighted nearest neighbors (WNN) graph (Figure 1A), skin cells were clustered using a standard Seurat pipeline (Figure 1B), and cell types were annotated. RNA counts were subsetted by tissue and cell type and then pseudobulked to aggregate expression across cells per gene, patient, and time point. A negative binomial GLM was fit using glmGamPoi, with time post-ASCT as the primary variable, adjusting for patient and library size. Differential gene expression was assessed using quasi-likelihood ratio tests.
Results: Monocytes (PBMC) and macrophages (skin) demonstrated the most robust transcriptomic changes over time. In PBMCs, the top differentially expressed gene was SERPINE1 (Plasminogen Activator Inhibitor-1), a known SSc biomarker (LFC -3.48, p = 7.86×10⁻⁶). Figure 1C shows longitudinal model fits of SERPINE1 in peripheral blood monocytes and skin macrophages, as well as expression feature plots across time points. Expression of LGMN, another fibrosis-associated gene, followed a similar trajectory (Figure 1D). Gene set enrichment analysis (GSEA) of gene expression downregulated post-ASCT identified pathways related to cell adhesion, monocyte chemotaxis, fibrinolysis, and cell differentiation (Figure 1E). Notably, RGCC, LGMN, and FOSL1, previously implicated in fibrotic and immune processes, were significantly downregulated in both PBMC and skin. These findings align with prior studies implicating macrophage-fibroblast crosstalk in adult SSc pathology.
Conclusion: Our integrated single-cell analysis reveals that ASCT results in significant reduction of fibrotic and inflammatory gene signatures in both blood and skin of jSSc patients. The longitudinal decrease in SERPINE1 and other SSc-associated genes supports ASCT’s role in molecular disease reversal. These transcriptomic findings, combined with parallel clinical improvement, underscore the therapeutic potential of immune reset strategies in modifying disease trajectory in jSSc.
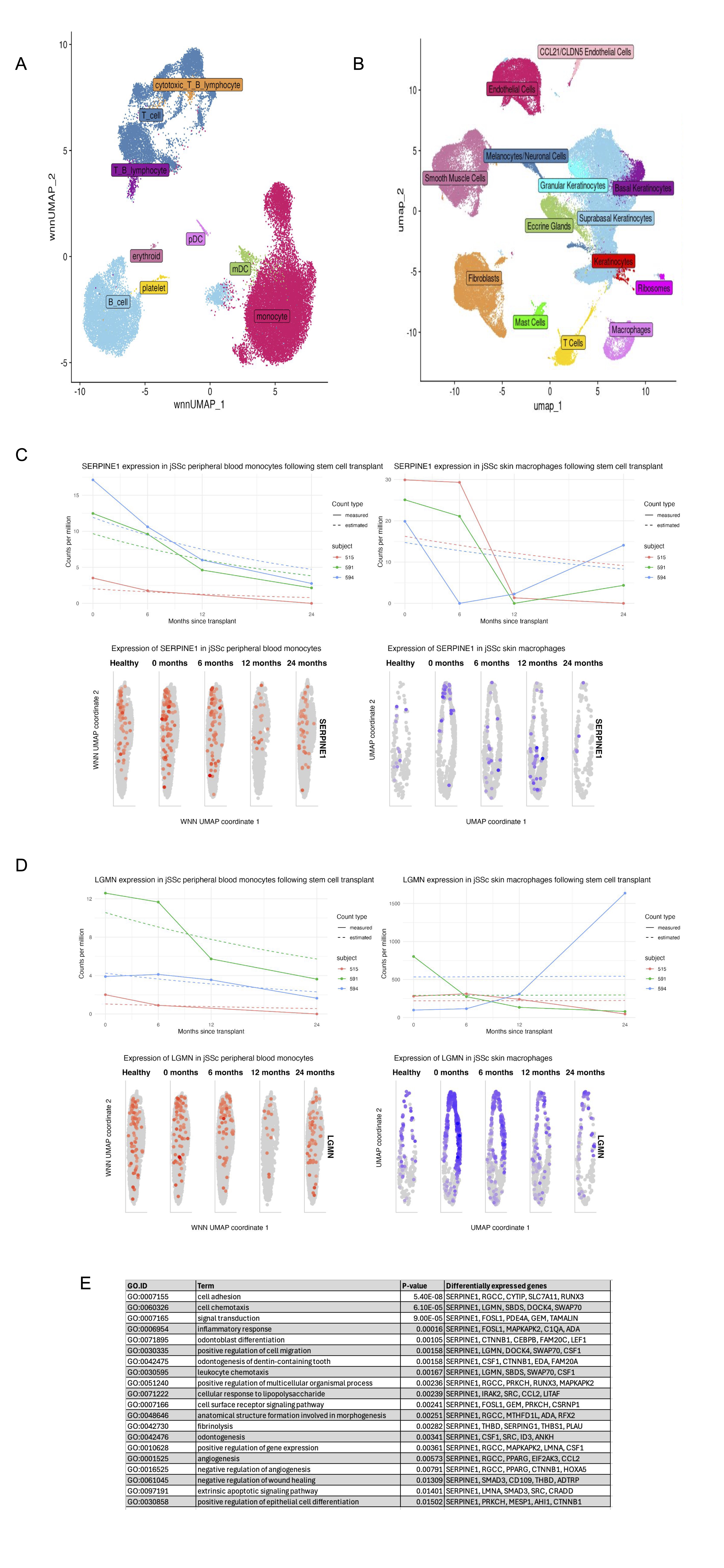 Figure 1. Longitudinal transcriptomic changes in paired peripheral blood and skin of juvenile systemic sclerosis (jSSc) patients following autologous stem cell transplant (ASCT)
Figure 1. Longitudinal transcriptomic changes in paired peripheral blood and skin of juvenile systemic sclerosis (jSSc) patients following autologous stem cell transplant (ASCT)
Legend:
A. Weighted nearest neighbor (WNN) UMAP of peripheral blood CITE-seq data from all time points, annotated by major immune cell type. B. UMAP of skin scRNA-seq data, annotated by major stromal and immune cell populations. C. SERPINE1 expression trajectories over time in peripheral blood monocytes (left) and skin macrophages (right). Top panels show log-linear longitudinal model fits for each patient, while bottom panels display SERPINE1 feature plots at each time point, including healthy controls and post-ASCT time points. D. Longitudinal expression and feature plots for LGMN, a fibrosis-associated gene, in peripheral blood monocytes and skin macrophages, using the same layout as panel C. E. Gene ontology (GO) enrichment analysis of genes downregulated following ASCT, highlighting biological processes such as cell adhesion, monocyte chemotaxis, fibrinolysis, and cell differentiation.
These results highlight dynamic and coordinated downregulation of profibrotic and inflammatory gene programs in skin and blood compartments post-ASCT, with SERPINE1 and LGMN among the top differentially expressed genes associated with disease resolution.
To cite this abstract in AMA style:
Elrod J, hutchins T, Sanyal A, Szabolcs P, Horvei P, Li J, Townes F, Torok K. Longitudinal model of paired peripheral blood CITE-seq and skin scRNA-seq data in juvenile systemic sclerosis (jSSc) patients following autologous stem cell transplant (ASCT) reveals reduced expression of SSc marker genes [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/longitudinal-model-of-paired-peripheral-blood-cite-seq-and-skin-scrna-seq-data-in-juvenile-systemic-sclerosis-jssc-patients-following-autologous-stem-cell-transplant-asct-reveals-reduced-expressio/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-model-of-paired-peripheral-blood-cite-seq-and-skin-scrna-seq-data-in-juvenile-systemic-sclerosis-jssc-patients-following-autologous-stem-cell-transplant-asct-reveals-reduced-expressio/
