Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: ANCA-associated vasculitis (AAV) causes kidney damage, leading to a spectrum of chronic kidney disease (CKD) and end-stage kidney disease (ESKD). Clinically distinct kidney function trajectory groups have been identified in an observational cohort. We sought to confirm these findings in the Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis (PEXIVAS) randomized controlled trial.
Methods: PEXIVAS assessed the effect of use of plasma exchange vs non-use and reduced- vs standard-dose glucocorticoids on risks of ESKD and death in AAV patients with estimated glomerular filtration rate (eGFR) < 50 mL/min/1.73 m2 or lung hemorrhage. We included participants with ≥ 2 eGFR measures within a trajectory observation period up to 36 m (one ±30 d of randomization). We applied group-based trajectory modeling to identify groups with similar longitudinal changes in kidney function (% of baseline eGFR) during the observation period. We used multinomial logistic regression to evaluate baseline clinical characteristics associated with group membership. Outcomes were assessed for up to 7 y.
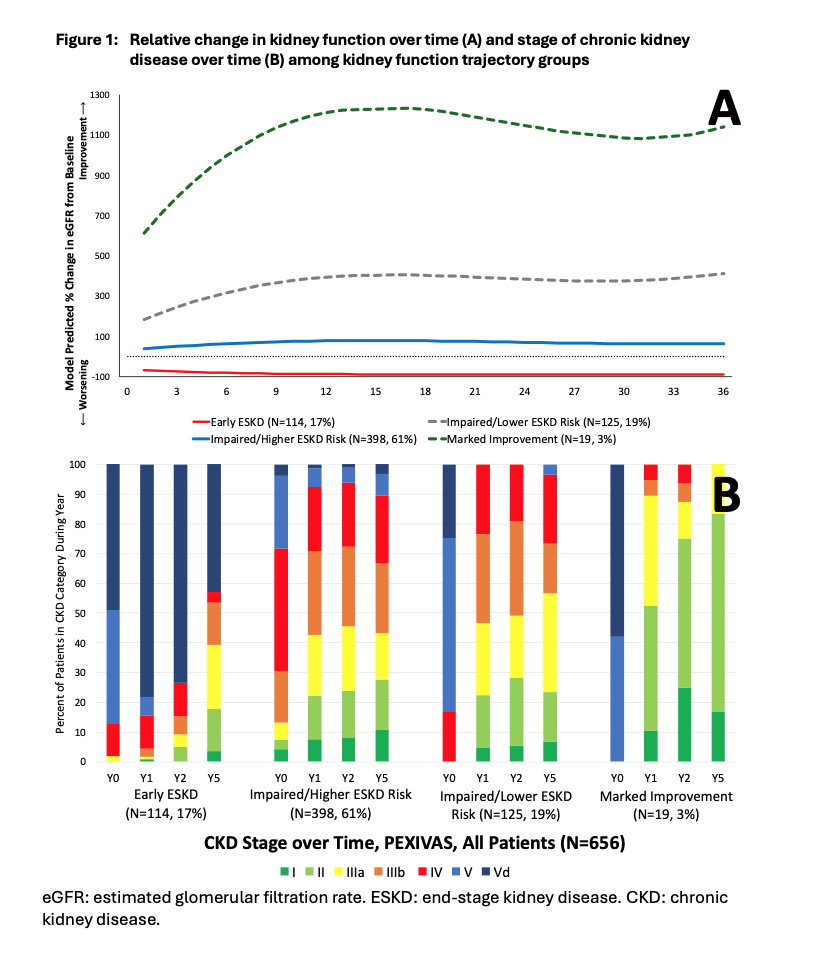
Results: We identified 4 trajectory groups among 656 participants with a median of 8 (IQR 6, 9) eGFR measurements: Group 1: Early ESKD, N=114 [17%]; Group 2: Marked Improvement, N=19 [3%]; Group 3: Impaired function/Higher ESKD risk, N=398 [61%]; and Group 4: Impaired function/Lower ESKD risk, N=125 [19%] (Figure 1A).
Kidney involvement was present in 650 (99%) participants. The median baseline eGFR was 15ml/min/1.73 m2. ESKD occurred in 136 (21%) patients, including 114 (100%) in the Early ESKD group, 21 (5%) in Impaired/Higher ESKD risk, and 1 (1%) in the Impaired/Lower ESKD risk group. Restricted mean survival time free from ESKD was 91 d in the Early ESKD group, 2318 days in Impaired/Higher ESKD risk, 2452 days in Impaired/Lower ESKD risk, and 2487 days in the Marked Improvement group. CKD stage IIIb or greater was most common in the Early ESKD group at 1, 2 and 5 years, less common in the Impaired/Higher ESKD risk and Impaired/Lower ESKD risk groups, and least common in the Marked Improvement group at all 3 timepoints (Figure 1B).
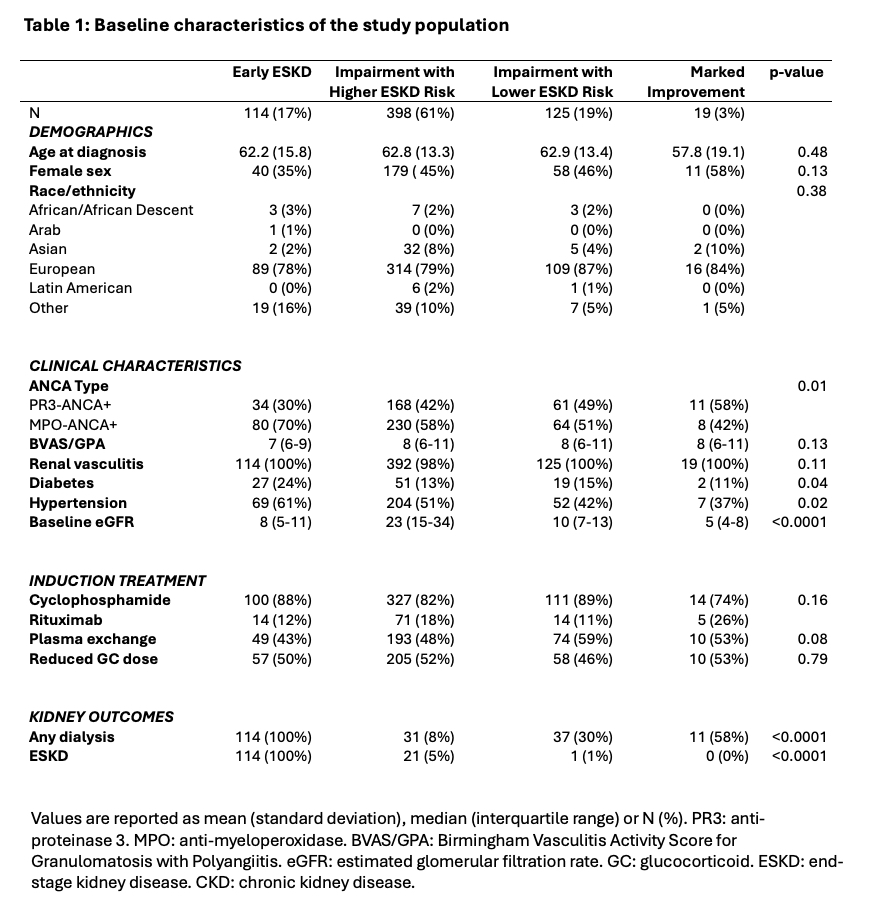
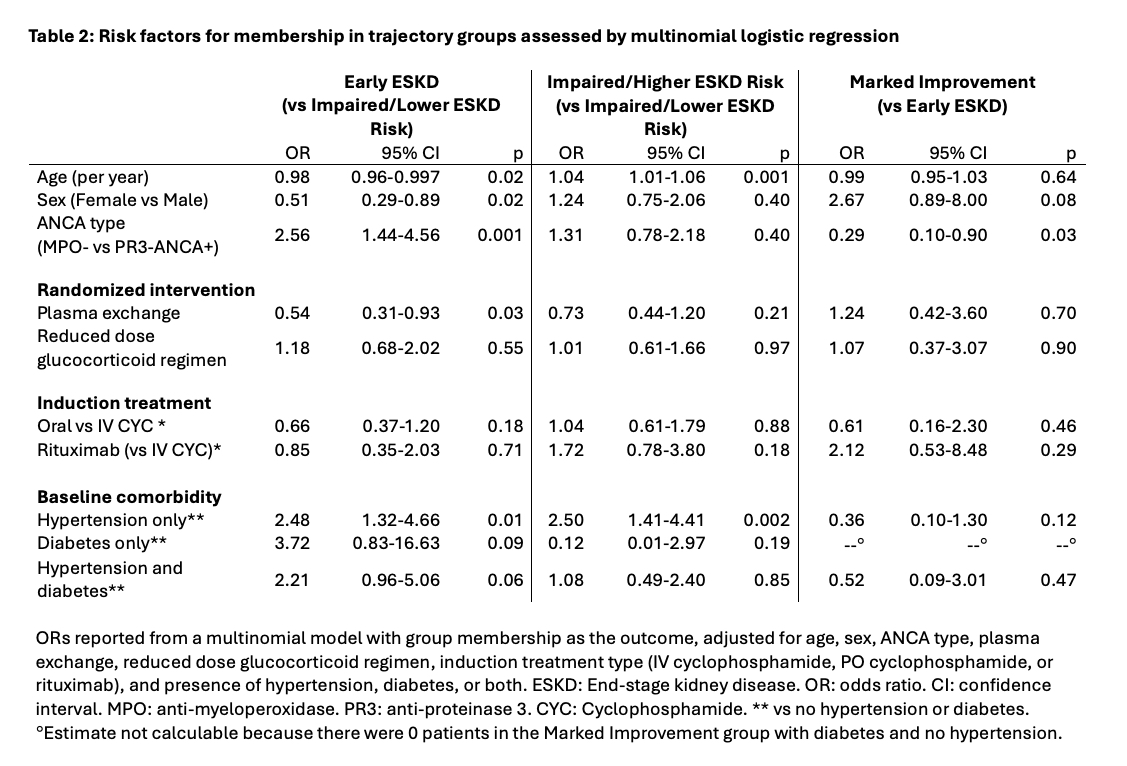
Age, sex, and ethnicity were similar between groups (Table 1). Hypertension and diabetes mellitus at baseline were most common in Early ESKD. Randomization to plasma exchange (OR 0.54; Table 2), younger age (OR 0.98 per y), female sex (OR 0.51), and higher baseline eGFR (OR 0.94 per 1mL/min/1.73 m2 increase) were associated with lower risk of Early ESKD vs Impaired/Lower ESKD risk group membership. In contrast, MPO-ANCA+ (OR 2.56) and hypertension (OR 2.48) were associated with higher risk of Early ESKD group membership. Older age, hypertension, and higher baseline eGFR were associated with higher risk of Impaired/Higher ESKD vs Impaired/Lower ESKD risk group membership (Table 2).
Conclusion: We identified 4 kidney function trajectory groups in the PEXIVAS cohort. Trajectory groups were distinguished by baseline characteristics as well as outcomes. The degree to which trajectory can be modified remains uncertain.
To cite this abstract in AMA style:
Hanberg J, Zhang Y, Kronbichler A, Matyjek A, McAlear C, Odler B, Uchida L, Geetha D, Hawley C, Jayne D, walsh M, Merkel P, Wallace Z. Longitudinal Kidney Function Trajectories in Patients Enrolled in the Plasma Exchange and Glucocorticoids in ANCA-Associated Vasculitis Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/longitudinal-kidney-function-trajectories-in-patients-enrolled-in-the-plasma-exchange-and-glucocorticoids-in-anca-associated-vasculitis-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-kidney-function-trajectories-in-patients-enrolled-in-the-plasma-exchange-and-glucocorticoids-in-anca-associated-vasculitis-trial/