Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: JAK inhibitors (JAKi) have shown promising early results in systemic sclerosis (SSc) patients, yet real-world data on their longitudinal effects across different disease domains remain limited. We aimed to explore the safety and intra-patient evolution of pulmonary, cutaneous, and articular parameters in a well-characterized cohort of SSc patients treated with JAKi, using standardized efficacy metrics over 12 and 24 months.
Methods: Ours was an international, multi-center longitudinal retrospective cohort study. Continuous variables were reported as means (SD) or medians (IQR) as appropriate and categorical variables were reported as counts (%). Incidence rates were calculated per 100 patient-years, and descriptive statistics were used for calcinosis and DU events. To assess treatment efficacy, we evaluated changes in FVC%, DLCO%, modified Rodnan skin score (mRSS), tender and swollen joint counts (TJC, SJC), digital ulcers (DU), and calcinosis status. Baseline was defined as the first available measurement at JAKi initiation; follow-up values were taken within a ±2-month window from 12 and 24 months. For each parameter, the primary outcome was the delta from baseline. Paired comparisons used the Wilcoxon signed-rank test. Effect size was quantified using standardized mean change (SMC). Analyses were performed in R (version 4.3.3).
Results: Among the 32 patients treated with the 4 avaliable JAK inhibitors, the median (IQR) follow-up time on treatment was 16.9 (10.3-31.8) months, with a range from 1.4 to 52.0 months, amounting to 52.8 patient-years. At 12 months, pulmonary function parameters (FVC% and DLCO%) remained stable over time. Although small positive changes were observed (SMC +0.22 for FVC and +0.23 for DLCO), neither reached statistical significance (p=0.10 and p=0.33, respectively) (Figure 1A and 1B). At 12 months, the median mRSS showed a significant improvement (p=0.030), with an SMC of -0.29 (Figure 1C). Similarly, both TJC and SJC showed decreases, (p=0.0002 and p=0.0012, respectively) with SMCs of -1.19 for TJC and -0.69 for SJC (Figure 1D and 1E). At 24 months, mRSS, TJC, and SJC showed numerical improvements which were marginally significant for joints domain (SMC: -0.21, -1.13, and -1.14; p=0.17, p=0.054, and p=0.058, respectively). FVC% and DLCO% remained stable with no significant changes. Among 11 patients with calcinosis at baseline, 5 (45.5%) showed improvement, 3 (27.3%) experienced worsening, and 3 (27.3%) remained stable during follow-up. During follow-up, 10 patients developed at least one episode of active DUs, corresponding to an incidence rate of 21 events per 100 patient-years. Among patients treated with glucocorticoids (n=15), a trend toward dose tapering over time was observed (-0.038 mg/month), although borderline significant (p=0.1).
Conclusion: In this real-life SSc cohort, JAK inhibitors were associated with significant improvements in skin and joint involvement. Importantly, pulmonary function remained stable over time, suggesting a potential disease-modifying effect across multiple clinical domains. A steroid sparing effect was noted, and with improvement in calcinosis seen in some treated patients.
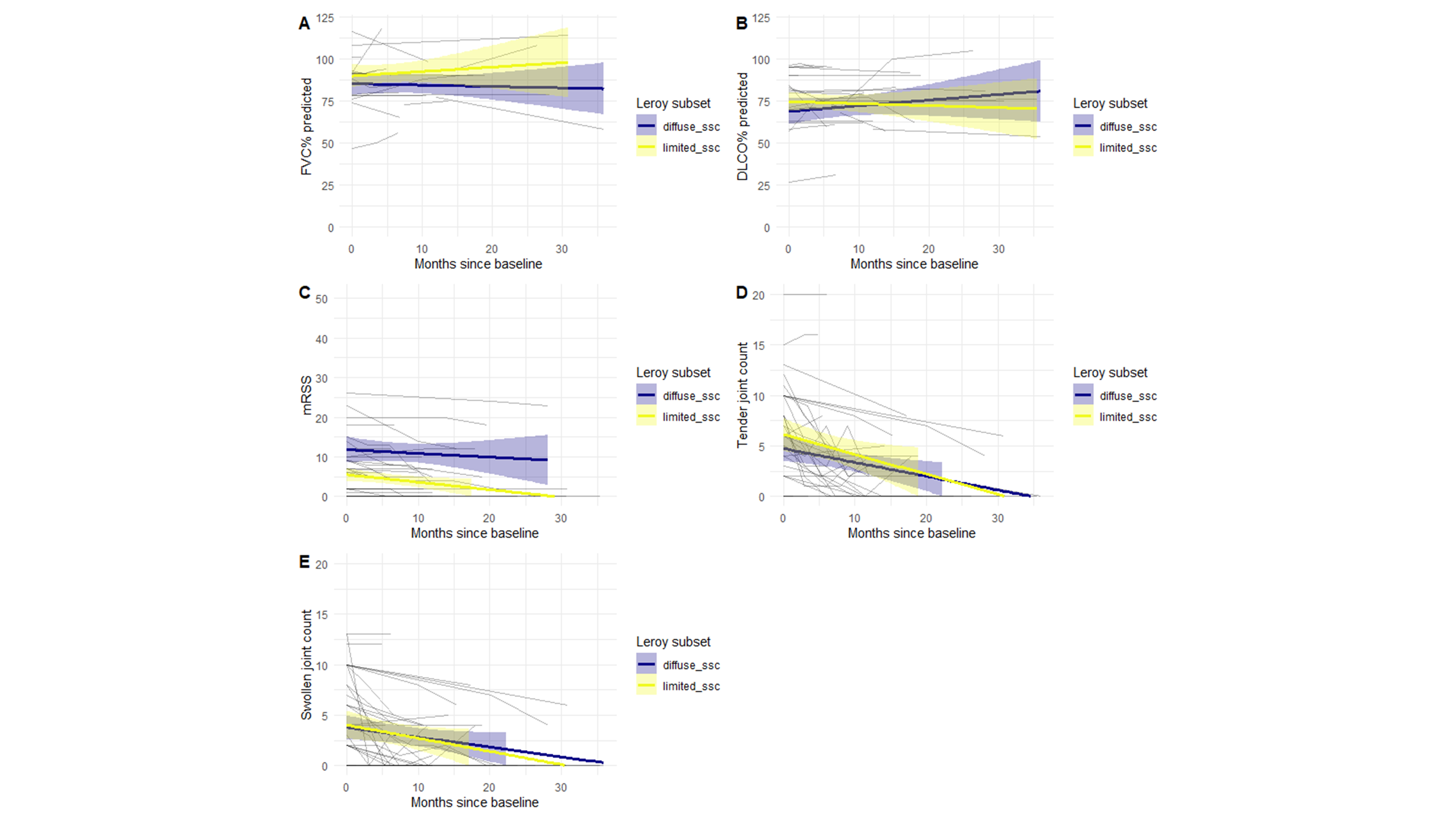 Table 1. Baseline clinical and demographic parameter of the JAK inhibitor-treated Systemic Sclerosis cohort.
Table 1. Baseline clinical and demographic parameter of the JAK inhibitor-treated Systemic Sclerosis cohort.
.jpg) Figure 1. Longitudinal changes in clinical parameters during JAK inhibitor treatment in systemic sclerosis. (A) FVC% predicted, (B) DLCO% predicted, (C) modified Rodnan skin score (mRSS), (D) tender joint count (TJC), and (E) swollen joint count (SJC), stratified by LeRoy subset. Each line represents an individual patient; solid lines and shaded areas represent linear trends with 95% confidence intervals.
Figure 1. Longitudinal changes in clinical parameters during JAK inhibitor treatment in systemic sclerosis. (A) FVC% predicted, (B) DLCO% predicted, (C) modified Rodnan skin score (mRSS), (D) tender joint count (TJC), and (E) swollen joint count (SJC), stratified by LeRoy subset. Each line represents an individual patient; solid lines and shaded areas represent linear trends with 95% confidence intervals.
To cite this abstract in AMA style:
Di Donato S, ALEGRE SANCHO J, Batalov A, Batalov Z, Bellando-Randone S, coccia c, de Pinto M, Giuggioli D, Hughes M. Longitudinal Clinical Response to JAK Inhibitors in Systemic Sclerosis: A Real-Life Multicentric Study Across Clinical Domains [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/longitudinal-clinical-response-to-jak-inhibitors-in-systemic-sclerosis-a-real-life-multicentric-study-across-clinical-domains/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-clinical-response-to-jak-inhibitors-in-systemic-sclerosis-a-real-life-multicentric-study-across-clinical-domains/
