Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: CD19-targeting chimeric antigen receptor (CAR) T-cell therapy has revolutionized treatment strategies for severe B-cell driven autoimmune diseases (AID) like Systemic Lupus erythematosus (SLE), Systemic Sclerosis (SSc) or Idiopathic Inflammatory Myositis (IIM). Cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS) and hematotoxicity are the most common and best described side effects, but little is known about AID-specific adverse events (1,2).Purpose of our study was to describe a new form of toxicity – Local Immune effector cell-Associated Toxicity Syndrome (LICATS), related to the treatment of patients with autoimmune disease (AID) with CD19-targeting chimeric antigen receptor (CAR) T-cells.
Methods: AID patients receiving CD19-CAR T-cell therapy in Erlangen and Duesseldorf with a follow-up of at least 30 days were assessed for organ-specific LICATS occurring after CAR T-cell infusion. Observed reactions were documented according to localization, time, duration and graded for severity (grade 1: spontaneous resolution; grade 2: glucocorticoid use; grade 3: glucocorticoid use plus prolonged hospital stay; grade 4: intensive care treatment). No systemic events, such as fever, chills, nausea, fatigue, diffuse pain/hypersensitivity or hemodynamic changes were classified as LICATS to separate it from CRS. Furthermore, per definition, LICATS were time-limited events that spontaneously regressed or stopped after a short course of glucocorticoid treatment in order to distinguish it from recurrence of AID.
Results: Between March 2021 and October 2024, 39 patients with AID were treated with CD19-CAR T-cells (20 systemic lupus erythematosus, SLE; 13 systemic sclerosis, SSc; 6 idiopathic inflammatory myositis, IIM). 25 patients (64.1%) were females. Median age was 36 years [22;43.5]. 54 local reactions, which we termed “Local Immune effector Cell-Associated Toxicity Syndrome” (LICATS) were recorded, affecting overall 30 patients (76.9%) with median (IQR) time of onset of 14 [5;21] days and a median duration of 11 [5;14] days. LICATS exclusively occurred during the B-cell aplasia phase and only involved organs previously affected by the respective AID. Most frequent affected organs were the skin (Nf19, 35.2%) and the kidneys (Nf12, 22.2%). Most LICATS were mild (grade 1: 35, grade 2: 16). Only three LICATS were grade 3. All LICATS resolved without sequelae.
Conclusion: LICATS is a new form of toxicity in CD19-CAR T-cell treated patients with AID, most likely based on the cleansing of immune cells from the affected organs. It is self-limited, organ-specific and usually mild in its intensity.References:< !1. Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease - A Case Series with Follow-up. N Engl J Med. 2024; 390:687-700.< !2. Isaacs JD. CAR T Cells - A New Horizon for Autoimmunity?. N Engl J Med. 2024; 390:758-759.
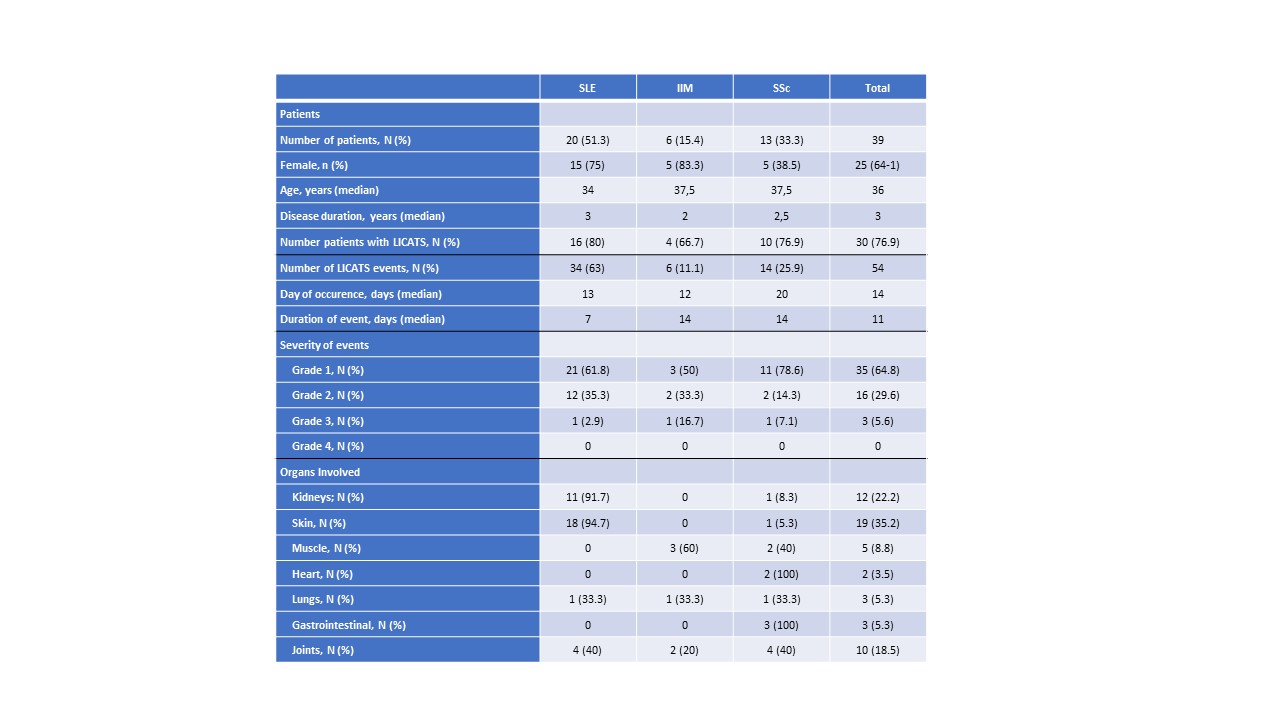 Demographics, Incidence and organ involvement of LICATS.
Demographics, Incidence and organ involvement of LICATS.
To cite this abstract in AMA style:
Hagen M, Müller F, Wirsching A, Kharboutli S, Spoerl S, Duesing C, Krickau T, Metzler M, Völkl S, Aigner M, Kretschmann S, Vasova I, Saake M, Schliep S, Kubacki T, Hunzelmann N, Bucci L, Taubmann J, Bergmann C, Györfi A, Dietrich S, Distler J, Grieshaber-Bouyer R, Mackensen A, Schett G. Local Immune effector Cell-Associated Toxicity Syndrome (LICATS) in CAR T-cell treated patients with Autoimmune Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/local-immune-effector-cell-associated-toxicity-syndrome-licats-in-car-t-cell-treated-patients-with-autoimmune-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/local-immune-effector-cell-associated-toxicity-syndrome-licats-in-car-t-cell-treated-patients-with-autoimmune-disease/
