Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Rheumatoid arthritis (RA) treatments are known to cause complex changes in lipids, in part by controlling disease activity. While there are robust data on increases in cholesterol associated with biologic therapies, e.g., tumor necrosis factor inhibitors (TNFi), there are limited data for triple therapy, methotrexate (MTX) + sulfasalazine + hydroxychloroquine. This study was carried out in the setting of the TARGET randomized active comparator trial that enrolled MTX inadequate responders with RA; all subjects underwent cardiovascular (CV) risk assessment with arterial FDG PET/CT at baseline and after 24 weeks. The objective of this study was to examine the changes in lipids pre/post-randomization to triple therapy or addition of TNFi. Further, we determined whether changes in lipid parameters differentially associated with CV risk as measured by arterial FDG PET/CT.
Methods: Subjects were randomly assigned to triple therapy or TNFi for 24 weeks. Baseline and follow-up measurements included disease activity score 28 (DAS28-CRP), lipid profile with total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and triglycerides (TG). CV risk was assessed with FDG-PET/CT scans at baseline and follow-up for change in arterial inflammation, measured as an arterial target-to-background ratio (TBR) in the most diseased segment (MDS) of the carotid artery or aorta. We tested differences in lipid levels within each treatment group over the 24-week study using paired t-tests. Linear regression models were constructed to test for difference in change in lipids by treatment arm adjusted by age, sex, (model 1) and change in DAS28-CRP (model 2). The association between changes in lipid parameters and changes in MDS TBR were tested using linear regression with and without treatment group as a covariate in the model; all analyses were adjusted by age and sex.
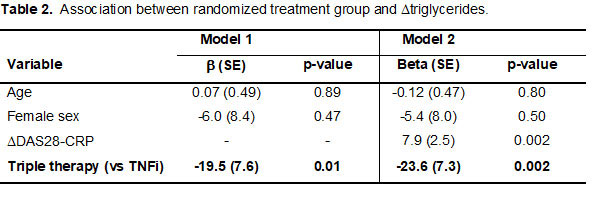
Results: We studied 112 subjects, mean age 60 years, 71% female, 57% seropositive, mean RA duration 5 years. Within the group randomized to triple therapy (n = 55), an increase in HDL-C was observed, while an increase in TC was observed within the TNFi group (n =57) (Table 1). Only change in TG differed significantly by treatment arm. On average, subjects on triple therapy had a 23.6mg/dL greater reduction in TG between baseline and follow-up compared to the TNFi arm, adjusting for age, sex (p=0.01); this association remained independent after adjusting for change in disease activity (p=0.002) (Table 2). In both unadjusted and adjusted models, changes in lipid parameters were not significantly associated with change in MDS TBR (all associations between change in lipid and change in MDS TBR, p >0.40).
Conclusion: In the TARGET trial, we observed modest lipid changes within treatment groups. When comparing between treatment groups, subjects randomized to triple therapy experienced a greater reduction in TG compared to TNFi, independent of disease activity. While subjects in the two treatment groups had differential changes in lipids, this variation was not associated with differential longitudinal changes in CV risk as measured by arterial inflammation.
To cite this abstract in AMA style:
Liao K, Giles J, Rist P, Glynn R, Santacroce L, Ridker P, Tawakol A, Bathon J, Solomon D. Lipids and Vascular Inflammation in Patients with RA Using Triple Therapy vs Methotrexate + TNFi: A Secondary Analysis of the TARGET Randomized Active Comparator Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/lipids-and-vascular-inflammation-in-patients-with-ra-using-triple-therapy-vs-methotrexate-tnfi-a-secondary-analysis-of-the-target-randomized-active-comparator-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lipids-and-vascular-inflammation-in-patients-with-ra-using-triple-therapy-vs-methotrexate-tnfi-a-secondary-analysis-of-the-target-randomized-active-comparator-trial/