Session Information
Date: Monday, November 9, 2020
Title: Pediatric Rheumatology – Clinical III: Systemic Autoimmune Disease (1988–1992)
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: Juvenile dermatomyositis (JDM) is a systemic autoimmune disease with a prominent interferon (IFN) signature. Treatment often requires prolonged high-dose steroids and other immunosuppressive medications. In a compassionate use program, we assessed efficacy and safety of baricitinib (JAK 1/2 inhibitor) in active refractory JDM.
Methods: Active (based on ≥3 core set measures (CSM)) and refractory (use of high dose steroids and ≥2 other medications, including ≥1 biologic therapy) patients with JDM were enrolled after washing out biologic agents other than IVIG. Baricitinib was dosed based on weight and renal function. Primary outcome was reduction in symptom daily diary score (DDS) of weakness, fatigue, musculoskeletal pain, and rash. Other assessments included International Myositis Assessment and Clinical Studies (IMACS) disease activity CSMs and Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI). STAT-1 phosphorylation (pSTAT1) and peripheral IFN markers (IFN-regulated gene score, CXCL10/IP-10) were assessed. Linear mixed models were used to compare measures to baseline. Safety and tolerability were assessed.
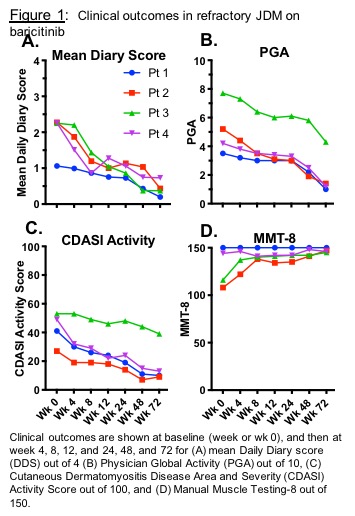
Results: Four paitents with JDM (5.8-20.7 years old) were enrolled (NCT01724580). Patients received baricitinib 4-12 mg/day PO divided BID. There were clinically relevant improvements up to 72 weeks after baricitinib. DDS changed from a mean of 2.0 (range 1.1-2.3) to 0.4 (0.2-0.7; 68-83% decrease, p< 0.01) (Fig 1A). Physician Global Activity visual analog scale (VAS) decreased from a mean of 5.2 (3.5-7.7) to 2.0 (1.0-4.3; 44-73% decrease, p< 0.01) (Fig 1B). Extramuscular Global Activity VAS decreased from mean 5.1 (3.0-7.3) to 1.8 (0.5-4.5; 38-83% decrease, p< 0.01). CDASI activity score reduced from mean 43 (27-53) to 18 (9-39; 14-36 point decrease, p< 0.01) (Fig 1C). In 2/4 pts with baseline weakness, manual muscle testing (MMT8) increased from 108 to 147 and 116 to 145 (mean improvement 24%) (Fig 1D). By the ACR-EULAR JDM response criteria, the Total Improvement Score at 72 weeks was mean 60.6, range 42.5-80 (1 minimal, 1 moderate, 2 major improvement). Three patients reduced prednisone: mean 13.7 (range 10-21) to 4 (3.5-4.5) mg/day; 1 patient remained on 5mg/day. IFN-alpha stimulated pSTAT1 in different immune cell subsets negatively correlated with plasma baricitinib levels. All IFN markers decreased. Baricitinib was generally well tolerated. There were no serious adverse events (AEs). Infections were the most common AE. No AEs required holding/discontinuing baricitinib.
Conclusion: Preliminary data on the use of baricitinib (JAK 1/2 inhibitor) in four patients with refractory JDM are encouraging, showing steady and prolonged improvement in symptom DDS as well as in validated disease activity measures, including both skin and muscle. All patients met clinically significant improvement by the ACR-EULAR response criteria. A corresponding decrease in pSTAT1 and IFN markers was observed. Baricitinib was generally well tolerated and further evaluation in JDM should be considered.
Disclosures: Baricitinib provided by Eli Lilly and Company, expanded access program sponsor. Other support: IRP of NIH, NIAMS, NIEHS, CC.
To cite this abstract in AMA style:
Kim H, Bergeron L, Dill S, O'Brien M, Vian L, Jain M, Manukyan M, Li X, Lu S, Tsai W, Mishra Thakur K, Shi Y, Gadina M, Brundidge A, Millwood M, Rider L, Colbert R. Janus Kinase (JAK) Inhibition with Baricitinib in Refractory Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/janus-kinase-jak-inhibition-with-baricitinib-in-refractory-juvenile-dermatomyositis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/janus-kinase-jak-inhibition-with-baricitinib-in-refractory-juvenile-dermatomyositis/