Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Sjögren’s disease (SjD) is an autoimmune disease affecting secretory glands and other organs with no approved systemic treatments. Iscalimab (CFZ533) is a mAb directed against CD40, a novel way to target underlying disease pathophysiology. TWINSS is an ongoing phase 2b dose-ranging trial assessing safety and efficacy of multiple CFZ533 doses in two distinct patient (pt) populations with SjD of moderate/severe systemic disease (Cohort 1/C1) and of low systemic disease and high symptom burden (Cohort 2/C2). We report Week 24 primary results from both cohorts (N=273).
Methods: In C1, 173 pts were randomized (1:1:1:1) to CFZ533 150 mg or 300 mg or 600 mg s.c. q2w or placebo (PBO). In C2, 100 pts were assigned (1:1) to CFZ533 600 mg or PBO. Key inclusion criteria were: fulfilled ACR/EULAR 2016 classification criteria; stimulated salivary flow rates of ≥0.1 mL/min; anti-Ro/SSA positivity; C1: EULAR Sjögren’s Syndrome (SS) Disease Activity Index (ESSDAI) ≥5 (on 8 selected domains), EULAR SS Patient Reported Index (ESSPRI) ≥5; and C2: ESSDAI < 5 with presence of activity in biologic domain ( >0), ESSPRI (fatigue or dryness ≥5, impact of dry eye on everyday life [IDEEL] ≥30). Statistical methods included Multiple Comparison Procedure – Modelling methodology to assess the dose response of the ESSDAI change from baseline (C1) and mixed model for repeated measures to estimate the adjusted mean change from baseline and difference vs PBO (C1 and C2). Similar methods were applied to all other endpoints including ESSPRI.
Results: Baseline characteristics were balanced across treatments except in C1 where more pts on iscalimab 300 mg had severe disease activity (ESSDAI >13; 44%) and used HCQ (70%), compared to the other treatment arms (ESSDAI >13, 19-28% and HCQ, 47-64%, respectively).
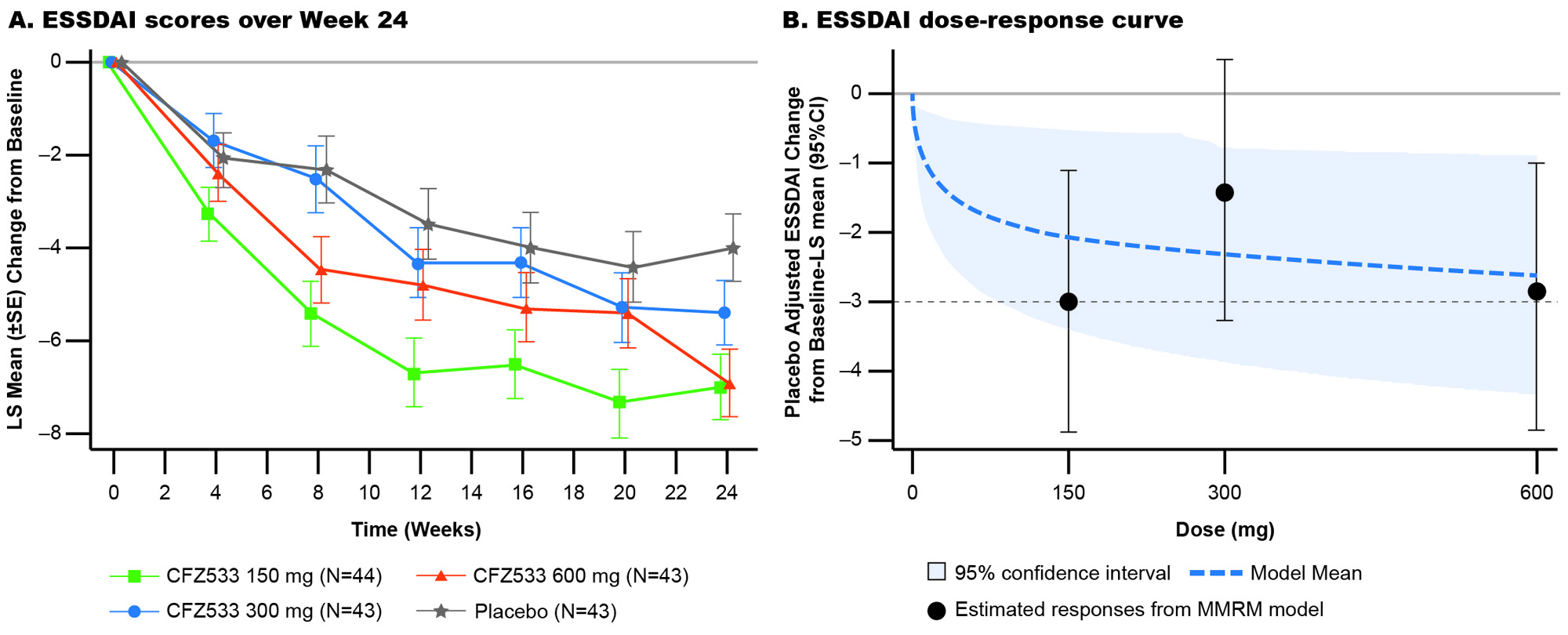
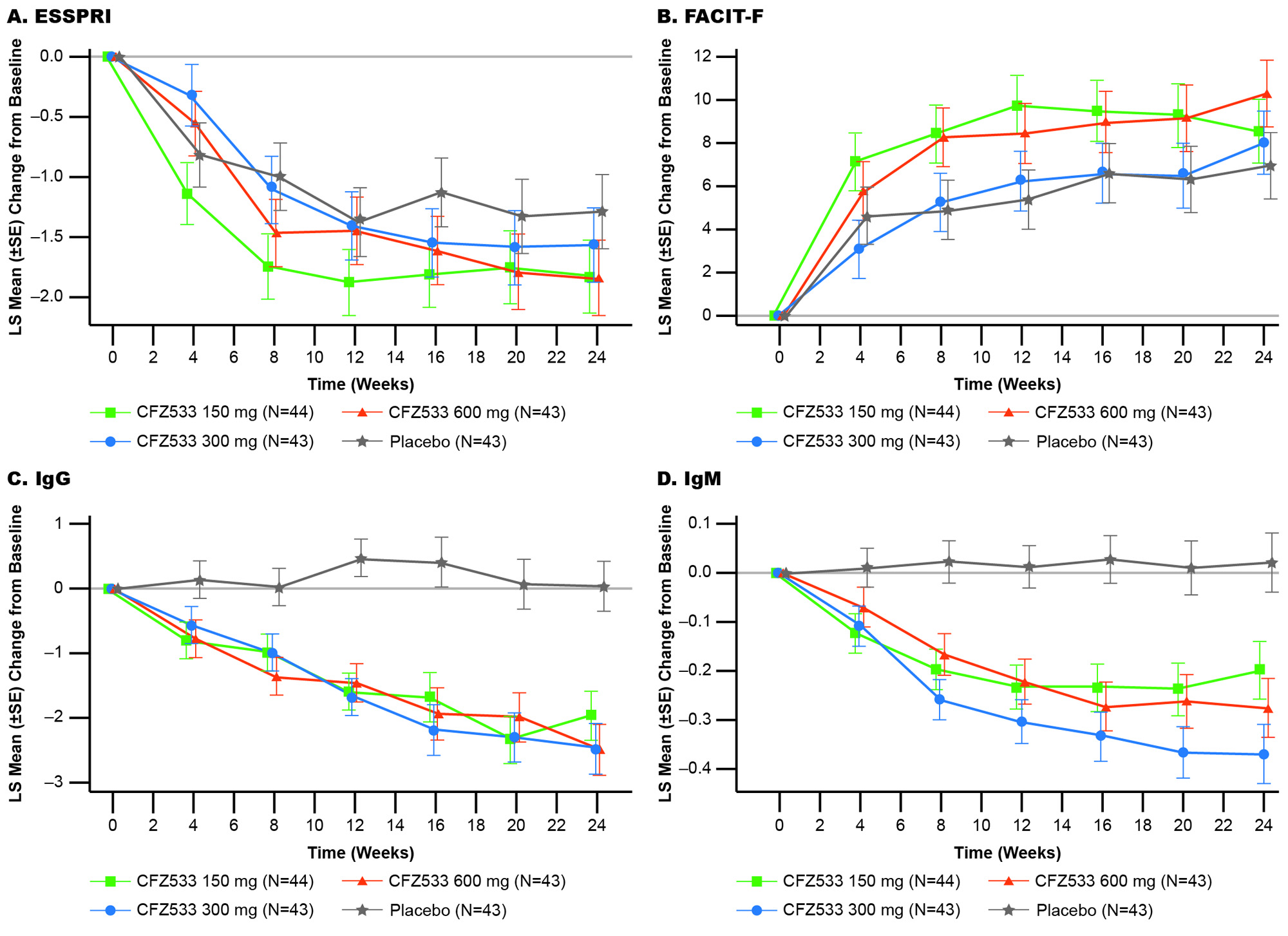
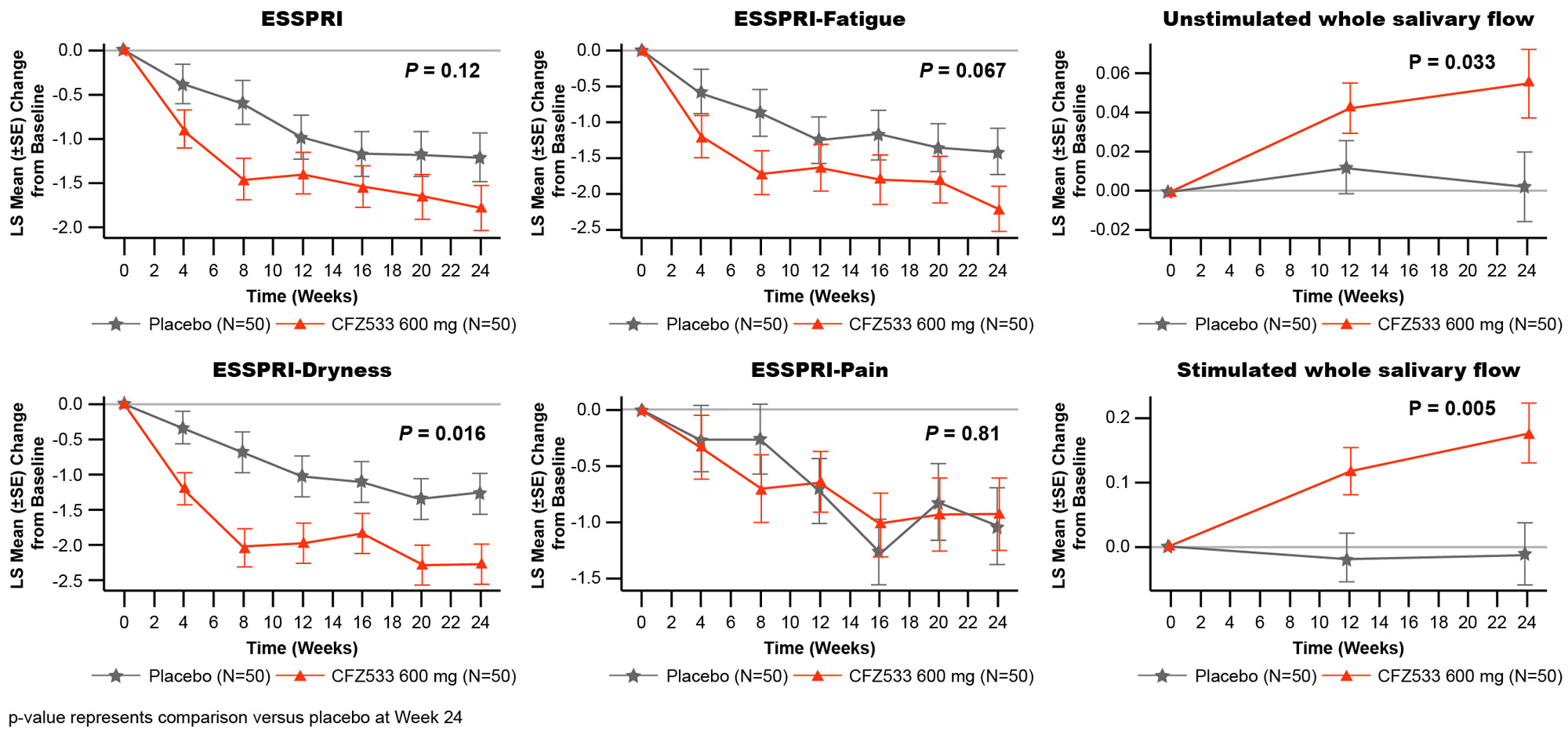
In C1, ESSDAI change from baseline at Week 24 showed statistically significant improvements compared to PBO for CFZ533 150 and 600 mg (least square mean difference (Δ) = –3.0 and –2.9; p < 0.005), and 300-mg dose showed a similar trend (Δ= –1.4; p = 0.16) (Fig 1). Primary objective of significant dose response of ESSDAI change from baseline at Week 24 (p = 0.004) with a log-linear dose-response was met. Predicted dose-response curve suggested that the drug effect plateaued at ≥150 mg. ESSPRI, FACIT-F (Fig 2), tear flow, and salivary flow (SF) rates showed numerical trends for improvement with active treatment. By Week 24, IgG/IgM as objective pharmacodynamic markers showed significant and similar plateaus of reduction for all doses of CFZ533 compared to PBO. In C2, the primary objective of ESSPRI change from baseline showed a strong trend (Δ = –0.57; p = 0.12) toward improvement with dryness (Δ = –1.0; p = 0.016) and fatigue (Δ = –0.8; p = 0.067) as the response drivers (Fig 3). For exploratory outcomes, statistically significant increases in SF were seen.
At Week 24, SAE rates in C1 (PBO, CFZ533 150 mg, 300 mg, 600 mg) were 2.3%, 2.3%, 7.1%, and 9.1%, respectively, in C2 (PBO or CFZ533 600mg) 4% each. Infection rates in C1 were 39.5%, 45.5%, 26.2%, and 43.2%; and in C2 42.0% and 56.0%, respectively.
Conclusion: Iscalimab showed a clinically important improvement over placebo in 2 distinct populations of SjD patients and was well tolerated without obvious safety signals.
To cite this abstract in AMA style:
A Fisher B, Mariette X, S Papas A, Grader-Beck T, Bootsma H, Ng W, Van Daele P, Finzel S, Elgueta S, Hermann J, McCoy S, Bookman A, Sopala M, Luo W, Scheurer C, Hueber W. Iscalimab (CFZ533) in Patients with Sjögren’s Disease: Week 24 Efficacy and Safety Results of a Randomized, Placebo-controlled, Phase 2b Dose-ranging Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/iscalimab-cfz533-in-patients-with-sjogrens-disease-week-24-efficacy-and-safety-results-of-a-randomized-placebo-controlled-phase-2b-dose-ranging-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/iscalimab-cfz533-in-patients-with-sjogrens-disease-week-24-efficacy-and-safety-results-of-a-randomized-placebo-controlled-phase-2b-dose-ranging-study/