Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriasis (PsO) and psoriatic arthritis (PsA) are associated with higher rates of obesity and cardiometabolic complications. Apremilast (APR) is an inhibitor of phosphodiesterase 4, which plays an important role in inflammation, lipolysis, and insulin signaling. APR treatment has been associated with weight loss and improvements in cardiometabolic and inflammatory biomarkers in clinical and real-world evidence studies. It is not known how APR mediates these effects. This meta-analysis aimed to identify proteins that are differentially expressed in plasma from APR- compared with placebo (PBO)-treated patients and to further investigate potential pathways impacted by APR.
Methods: Plasma samples from 3 clinical trials (PALACE-1 [NCT01172938], active PsA; ADVANCE [NCT03721172], mild to moderate plaque PsO; DISCREET [NCT03777436], moderate to severe genital PsO) were analyzed. Only patients treated with the 30 mg BID dose of APR were included. SomaScan assay v4.1 was used to measure ~7000 protein analytes. Log-transformed change from baseline at Week 16 (W16) was calculated and differentially expressed proteins from APR- relative to PBO-treated patients were identified using two-sample t-test without adjusting for disease subtype. Multiple testing was corrected using the false discovery rate (FDR) approach with significance set at an FDR< 0.05. Analyses were performed for all patients and for a subgroup of patients with BMI ≥25 kg/m2 (overweight or obese). We also assessed the effect of APR on 15 SomaSignal® tests for cardiometabolic phenotypes and disease risk.
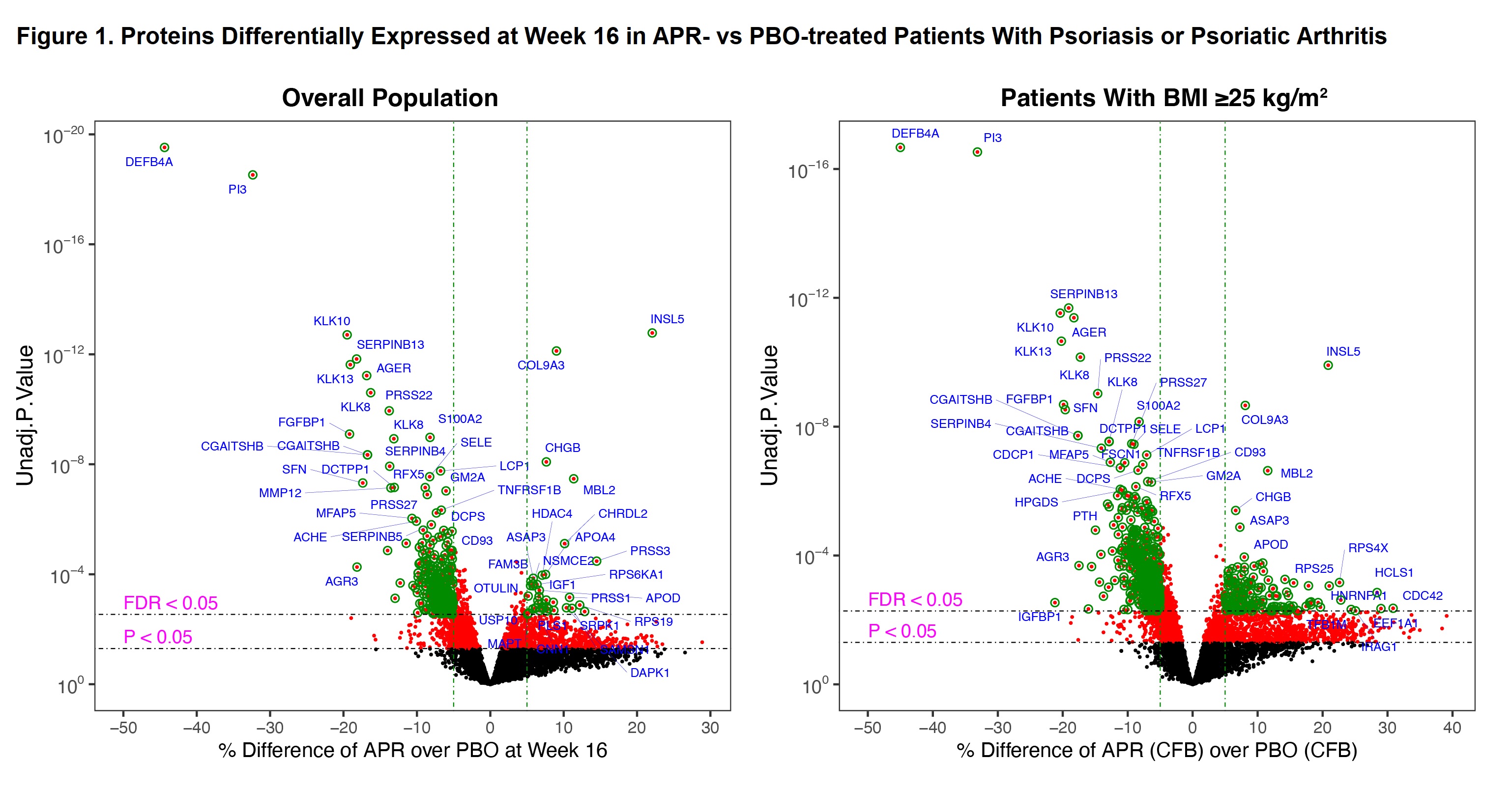
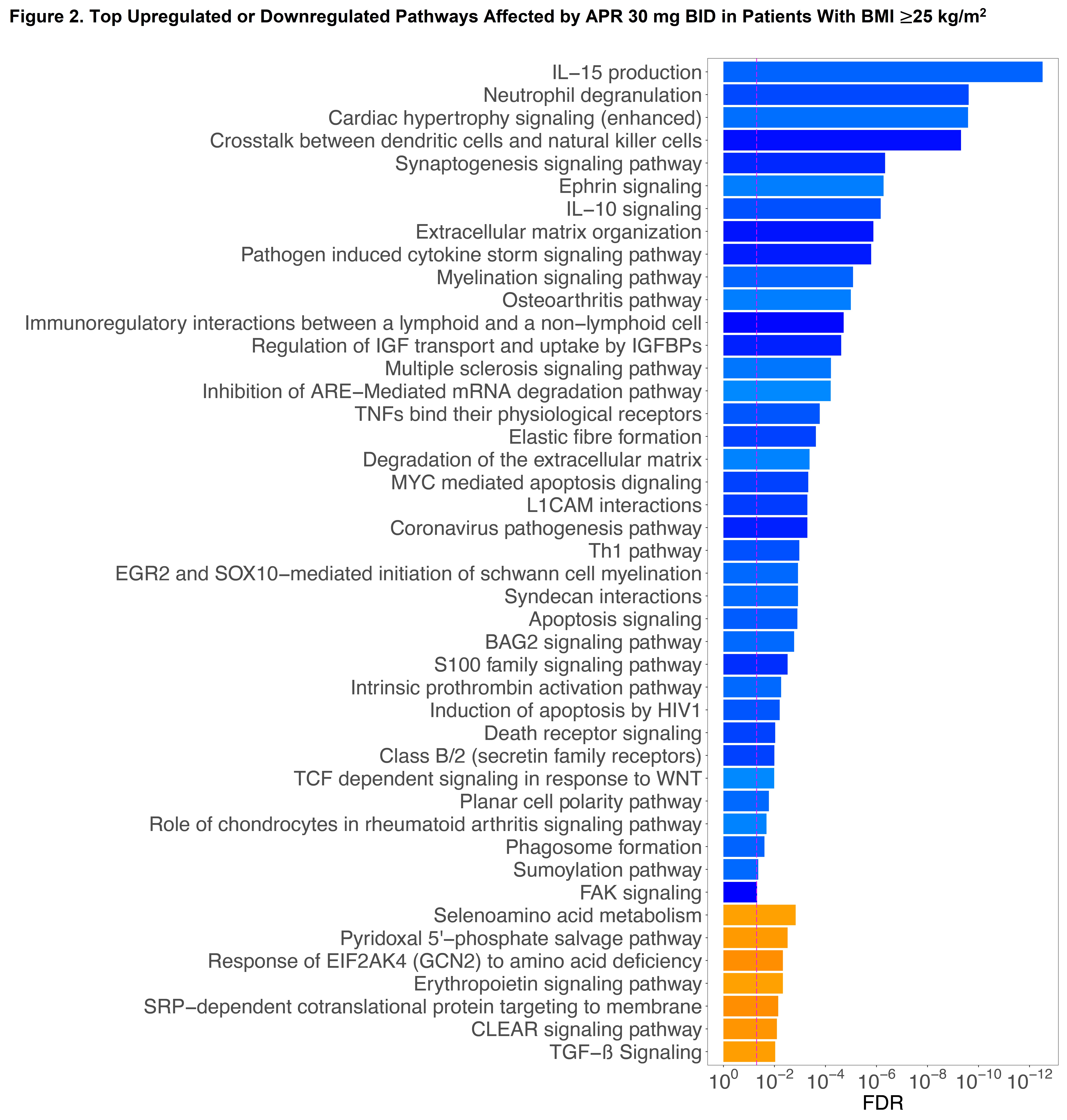
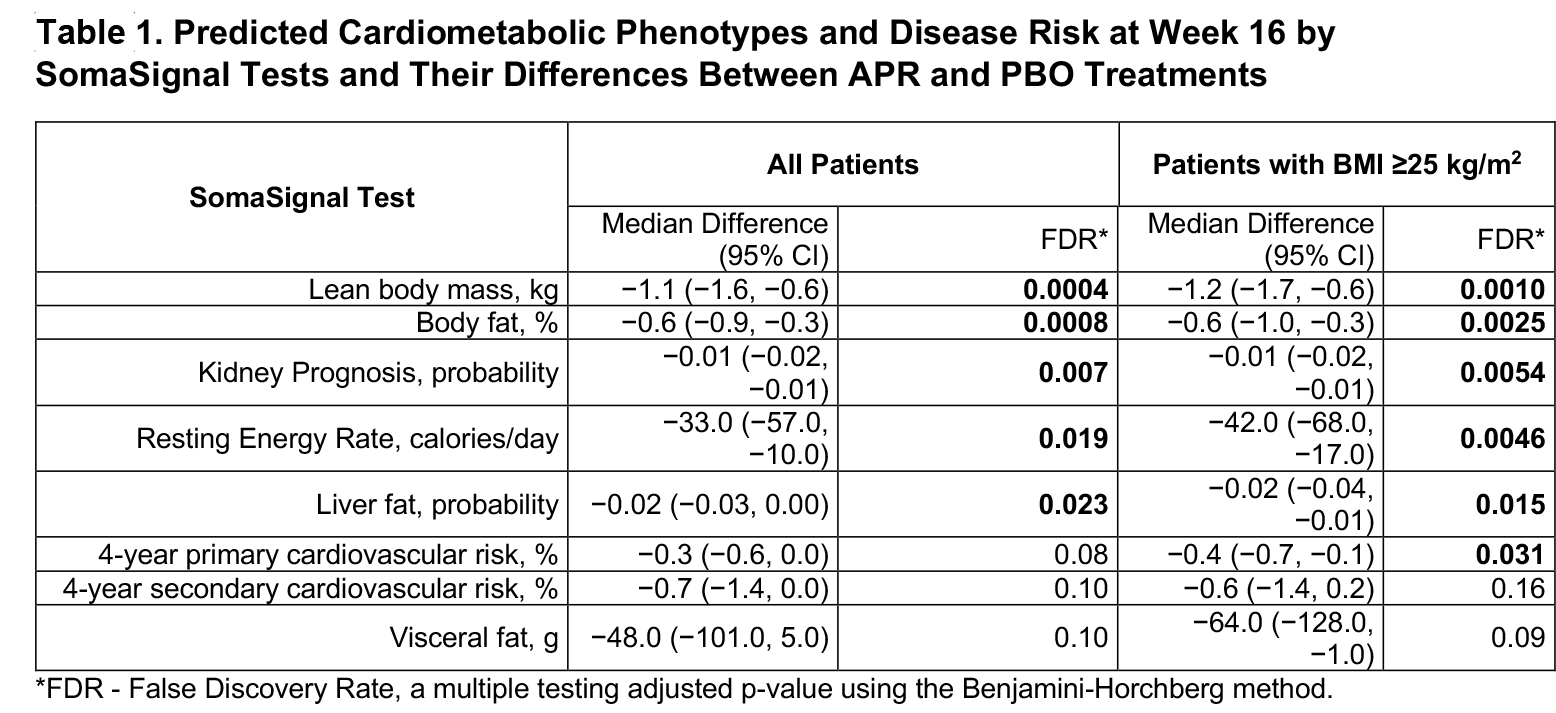
Results: We analyzed plasma from 268 APR and 219 PBO patients. Baseline parameters were balanced between APR and PBO arms. Relative to PBO, APR treatment resulted in 269 differentially expressed proteins at W16 in the overall population and 504 in the overweight/obese subgroup (Figure 1). APR modulated many metabolic-related proteins, including several involved in lipoprotein metabolism, regulation of food intake, and mitochondrial function. APR upregulated several metabolic-related pathways such as CLEAR signaling and pyridoxal 5′-phosphate salvage pathways (Figure 2). Consistent with our current understanding of its known mechanism of action, APR treatment inhibited multiple inflammatory and immune response-related proteins and pathways. Six cardiometabolic phenotypes and disease risks were significantly different between APR and PBO in overweight/obese patients (Table 1).
Conclusion: This meta-analysis is the first to investigate changes in protein expression after APR treatment at the broad molecular level to capture potential mechanisms by which APR mediates its cardiometabolic effects. In line with observations of weight loss and changes in other cardiometabolic parameters in previous studies, our findings show that APR modulated many metabolic-related proteins, pathways, metabolic phenotypes, and disease risks.
FDR – False Discovery Rate, a multiple testing adjusted p-value using the Benjamini-Horchberg method.
ARE, AU-rich elements; BAG, Bcl_2 associated athanogene; CLEAR, Coordinated Lysosomal Expression and Regulation; EGR, early growth response; EIF2AK4, eukaryotic translation initiation factor 2 alpha kinase 4; FAK, focal adhesion kinase; HIV, human immunodeficiency virus; IGF, insulin-like growth factor; IGFBP, IGF-binding proteins; L1CAM, L1 cell adhesion molecule; SOX10, SRY-box transcription factor 10; SRP, signal recognition particle; TCF, T-cell factor; Th, T helper; TGF, transforming growth factor; TNF, tumor necrosis factor.
To cite this abstract in AMA style:
Setiadi A, Burhans M, Hu X, Colgan S, Notari K, Teixeira dos Santos M, Kontzias A, Cheng S, Mehta N. Investigation of the Cardiometabolic-related Mechanism of Action of Apremilast Through Plasma Proteomics Profiling of Patients with Psoriasis or Psoriatic Arthritis from Three Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/investigation-of-the-cardiometabolic-related-mechanism-of-action-of-apremilast-through-plasma-proteomics-profiling-of-patients-with-psoriasis-or-psoriatic-arthritis-from-three-phase-3-studies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/investigation-of-the-cardiometabolic-related-mechanism-of-action-of-apremilast-through-plasma-proteomics-profiling-of-patients-with-psoriasis-or-psoriatic-arthritis-from-three-phase-3-studies/