Session Information
Date: Sunday, November 12, 2023
Title: Plenary I
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Pulmonary arterial hypertension (PAH) is a serious complication in SLE patients, with rapid progression and poor prognosis. In China, approximately 3% of the over one million SLE patients suffer from PAH. PAH is believed to result from inflammation and genetic abnormalities leading to dysfunction of pulmonary arterial endothelial cells (PAEC), followed by cellular proliferation and progressive pulmonary vascular remodeling. Considering the high morbidity and mortality rates, our research aims to investigate the pathogenesis of SLE-PAH and identify new biomarkers.
Methods: To investigate the pathogenesis of SLE-PAH, we employed various approaches. First, we performed whole-exome sequencing (WES) on peripheral blood samples from 150 SLE-PAH patients and conducted a genome-wide association study (GWAS) by comparing them with samples from 934 healthy controls. Subsequently, we validated mutations of candidate gene in 77 patients using Sanger sequencing. Additionally, we assessed the transcriptional expression levels of the identified genes in peripheral blood using RT-qPCR. In vitro intervention experiments were performed on human PAEC to elucidate the potential pathogenesis. RNA-seq and gene ontology analysis were employed to identify downstream pathways. Furthermore, we established SLE-PAH mouse models through pristane injection and hypoxia induction and measured pulmonary arterial pressure (PAP) using right heart catheterization. We also examined the effects of tail-intravenous injection of therapeutic vectors based on candidate gene. Lastly, we examined expression of candidate genes on monocrotaline (MCT) induced rat model of PAH.
Results: 1. Based on WES and GWAS, we identified the tumor necrosis factor receptor-associated factor 5 (TRAF5) as a susceptible gene in SLE-PAH. Transcriptional analysis revealed a significant reduction in TRAF5 expression in the peripheral blood of SLE-PAH patients, indicating its potential clinical diagnostic value. 2. Knockdown of TRAF5 in PAEC demonstrated notable effects on early apoptosis and the pathogenesis of PAH through distinct pathways. Flow cytometry analysis showed a significant increase in early apoptotic cells, and wound healing experiments demonstrated the loss of migratory ability in PAEC following TRAF5 knockdown. RNA-seq and KEGG pathway analysis identified TRAF5’s regulatory role in the TGF-beta pathway. 3. Our SLE-PAH mouse model successfully exhibited lupus phenotypes and increased mean PAP levels. Tail-intravenous injection of a TRAF5-overexpression vector attenuated PAH symptoms. Additionally, TRAF5 expression was significantly decreased in lung tissue of the MCT-induced PAH model rats.
Conclusion: In conclusion, lack of TRAF5 triggers the pathogenesis of PAH in SLE patients by inducing dysfunction of PAEC, which highlights the significance of TRAF5 as a susceptible gene in SLE-PAH and underscores its potential as a candidate biomarker for the diagnosis and therapy of SLE-PAH. Therefore, further research on TRAF5 and its involvement in the pathogenesis of SLE-PAH may provide valuable insights for the identification of novel therapeutic targets and improved management strategies for this condition.
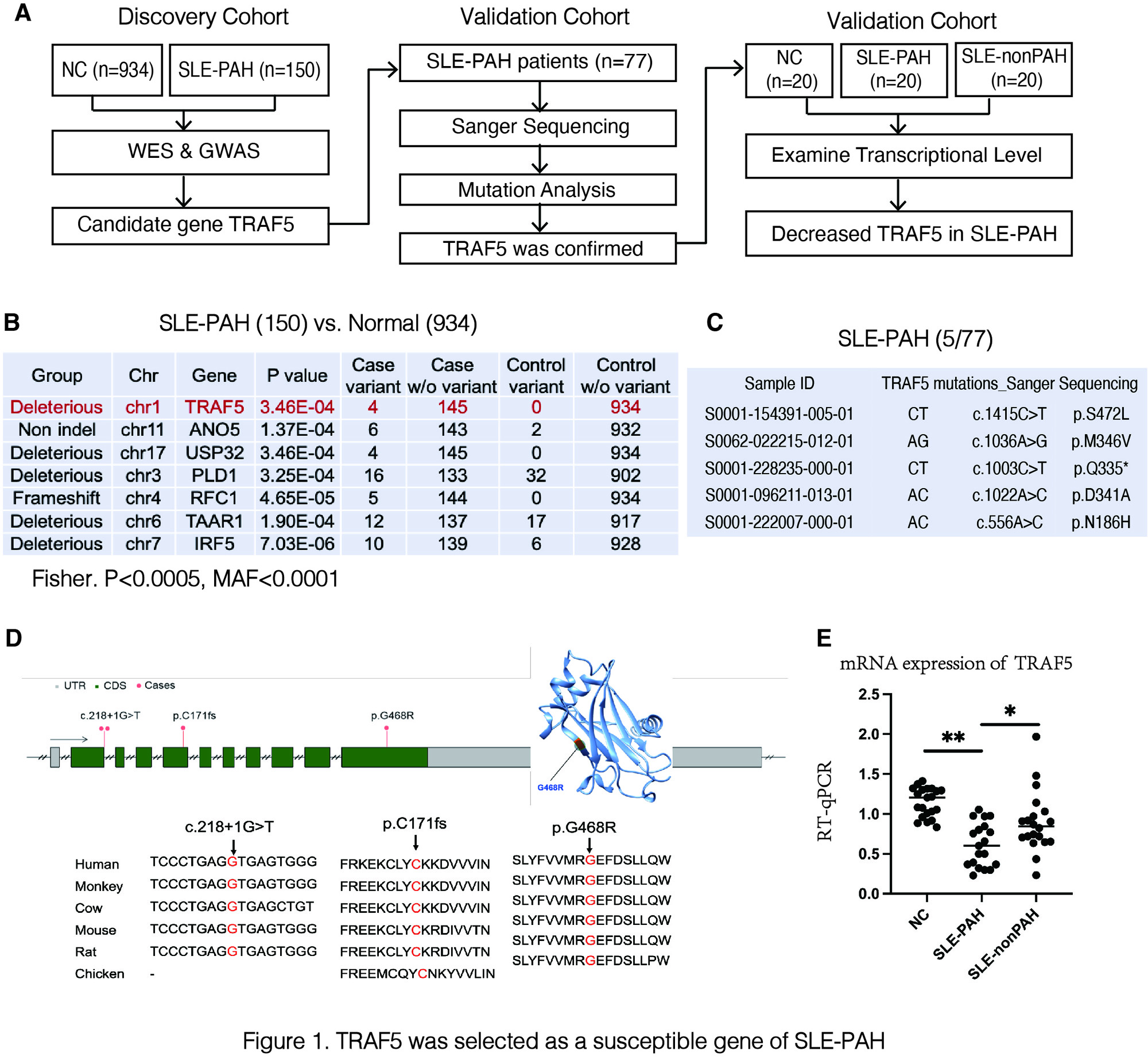
A) Workflow of selecting susceptible genes of SLE-PAH; B) and C) Results of GWAS and Sanger sequencing; D) Genomic and protein structure of TRAF5. p.G468R mutation causes dysfunction of protein. E) TRAF5 mRNA expression levels in peripheral blood .
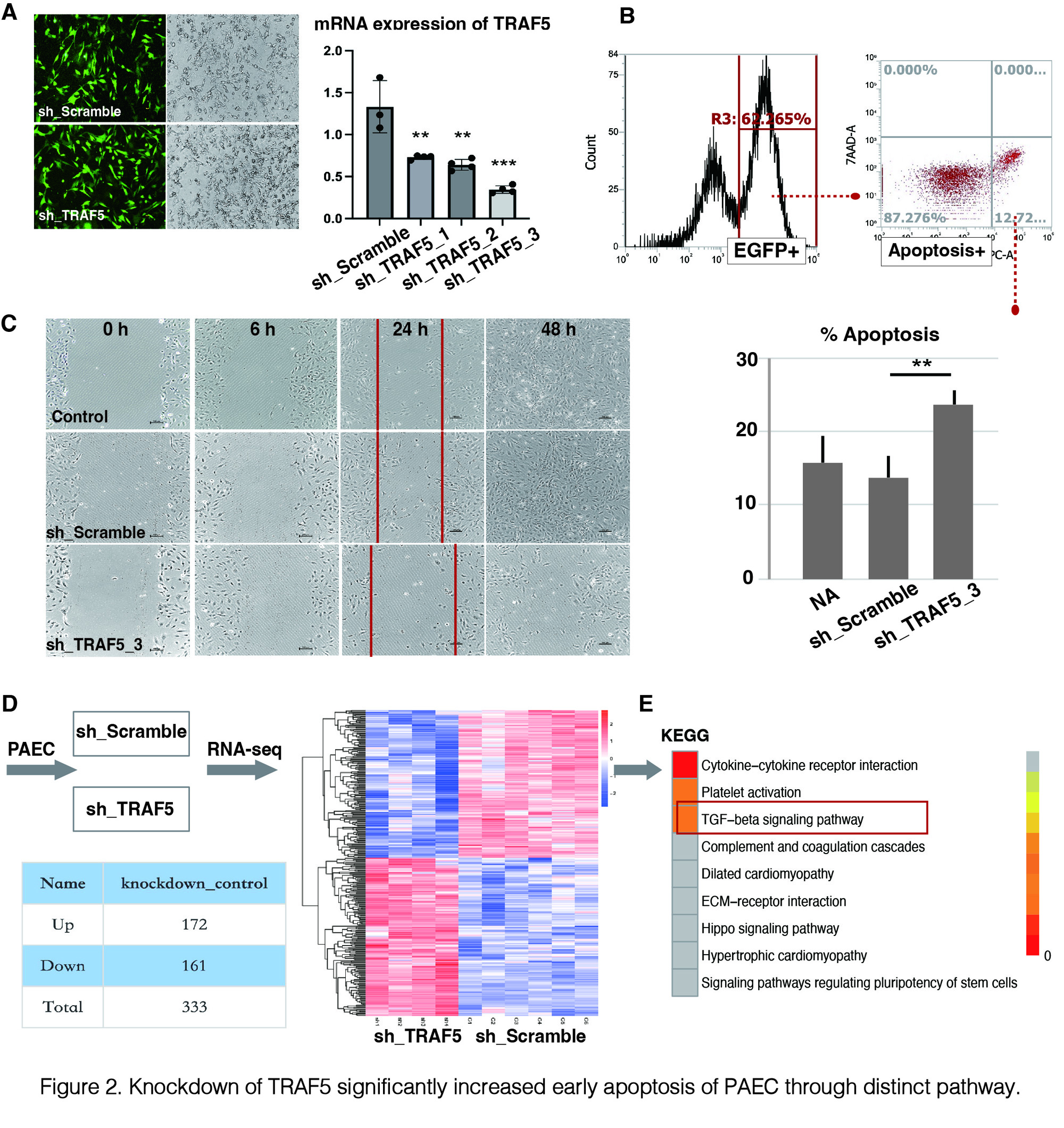
A) shTRAF5 transfected human PAEC (EGFP positive); B) FACS was performed to detect early apoptotic cells labeled with Annexin V-APC (Apoptosis Detection Kit); C) Wound healing experiments were performed in different groups; D) and E) TGF-β pathway was identified as downstream targets of TRAF5 using RNA-seq and KEGG analysis.
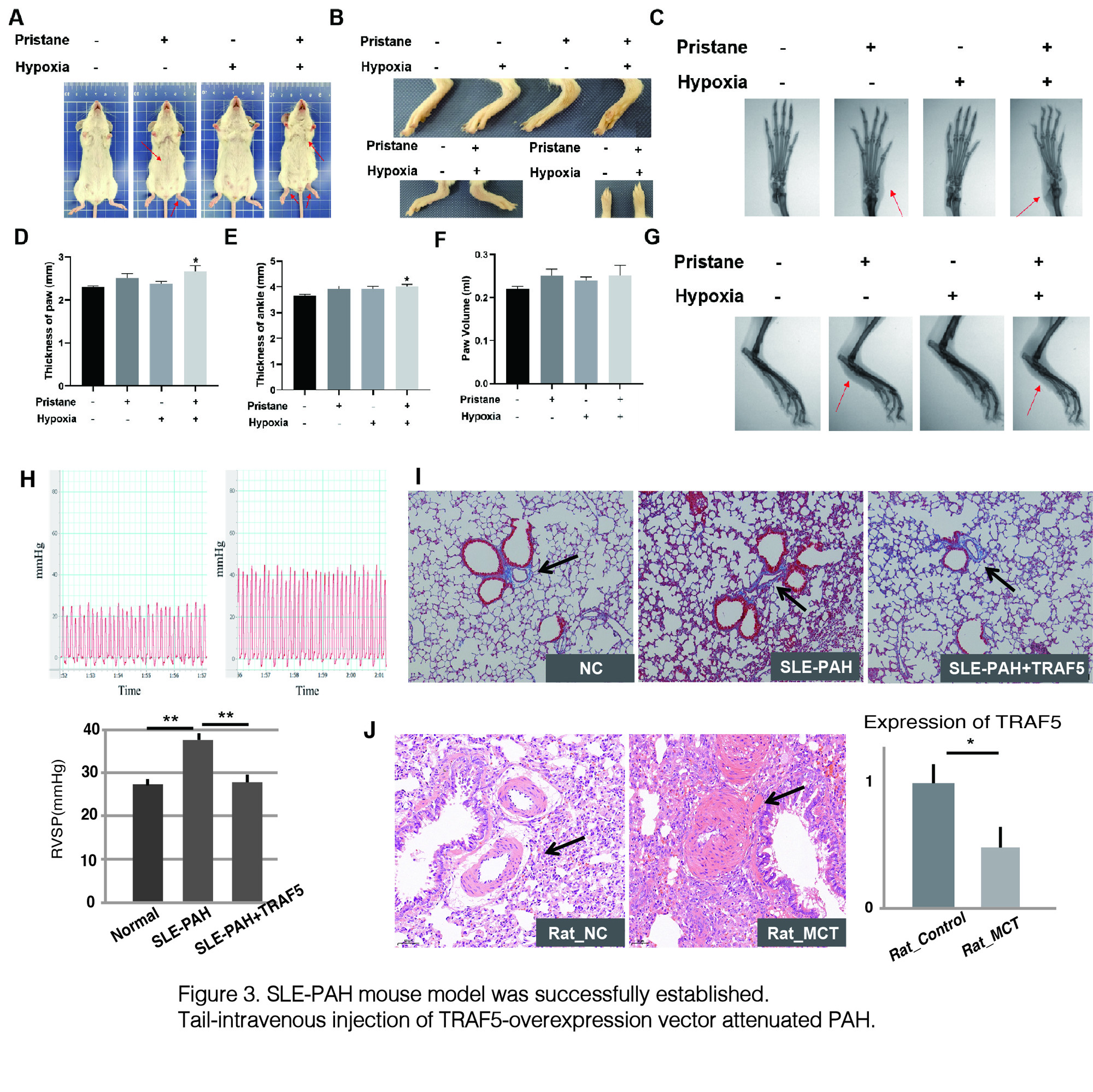
A-G) SLE phenotypes were observed after pristane induction; H-I) mPAP was elevated in model mouse, and tail-intravenous infection of TRAF5-overexpression vector attenuated PAH; J) Expression of TRAF5 was decreased significantly in MCT induced rats.
To cite this abstract in AMA style:
Deng X, Qian J, Wang R, ZHAO J, Wang Q, Yuan T, LI M, Zeng X. Investigating the Effects and Molecular Mechanisms of TRAF5 on the Pathogenesis of SLE Associated Pulmonary Arterial Hypertension [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/investigating-the-effects-and-molecular-mechanisms-of-traf5-on-the-pathogenesis-of-sle-associated-pulmonary-arterial-hypertension/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/investigating-the-effects-and-molecular-mechanisms-of-traf5-on-the-pathogenesis-of-sle-associated-pulmonary-arterial-hypertension/