Session Information
Date: Tuesday, November 14, 2023
Title: (1913–1944) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) are a heterogeneous group of diseases characterised mainly by the presence of inflammation and muscle weakness. The main subtypes of IIM are Dermatomyositis (DM), Polymyositis (PM), Inclusion body myositis (IBM), Immune-mediated necrotising myopathies (IMNM) and Anti-synthetase syndrome (ASS).
Currently the treatment of IIMs is not well established. Intravenous immunoglobulins (IVIG) are a therapeutic alternative for the treatment of IIM. Although their mechanism of action is not precisely known, several studies suggest that they are effective in the treatment of multiple rheumatological diseases, including IIM.
The main objective of this study is to describe the use of IVIG, as well as to determine its efficacy in different subtypes of IIM in a cohort of patients from northern Spain.
Methods: Observational study of patients with IIM who required at least one cycle of IVIG between January 2000 and December 2022. Patients with IIM who met a) EULAR/ACR 2017 criteria for DM and PM, b) European Neuromuscular International Workshop 2016 definition for MNIM and c) Connor’s criteria in SAS were included.
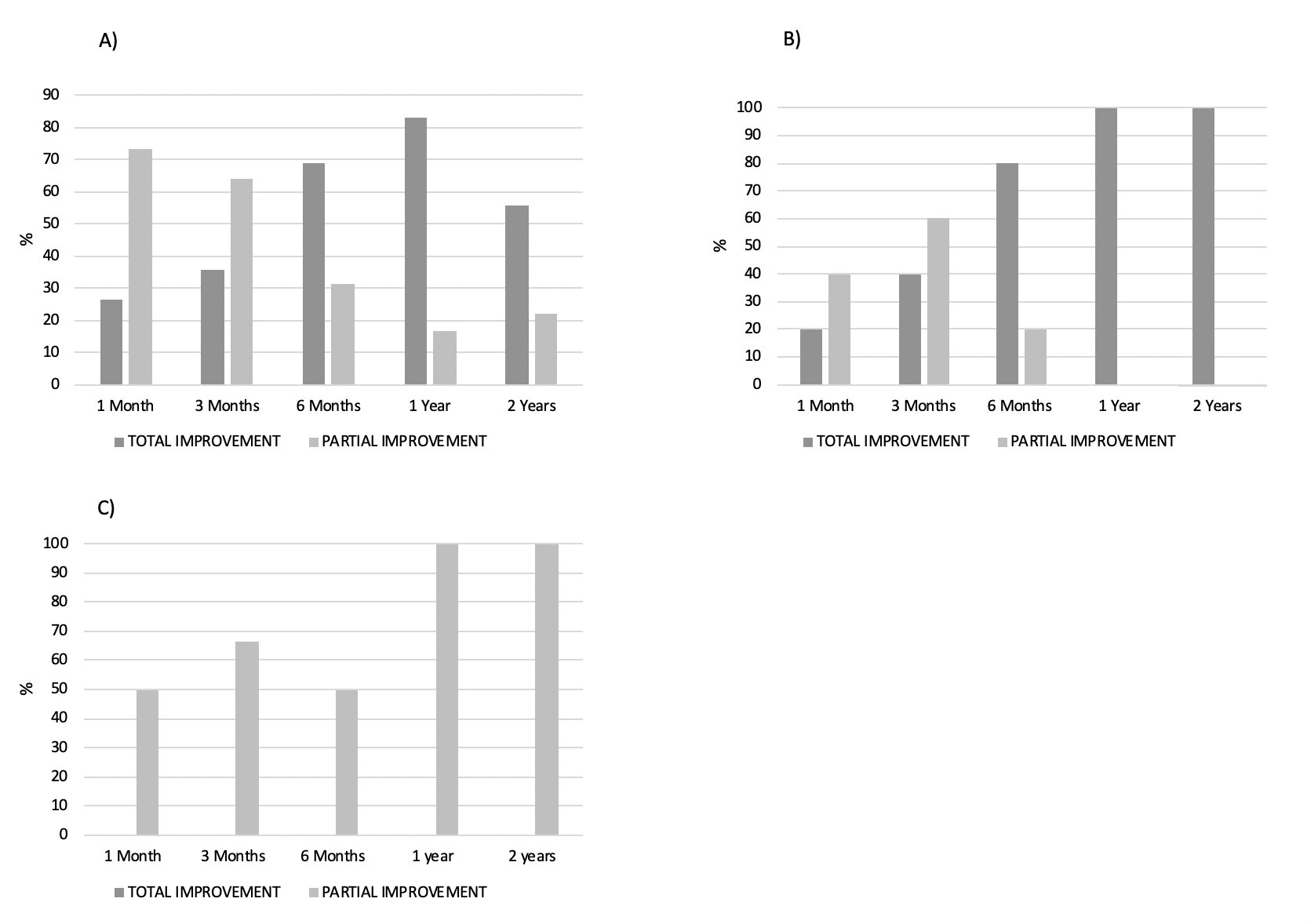
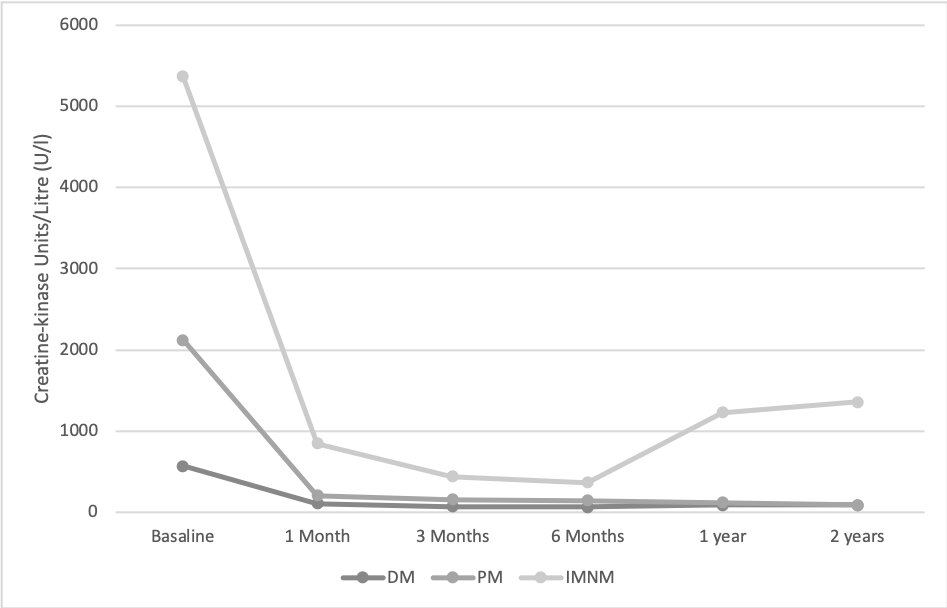
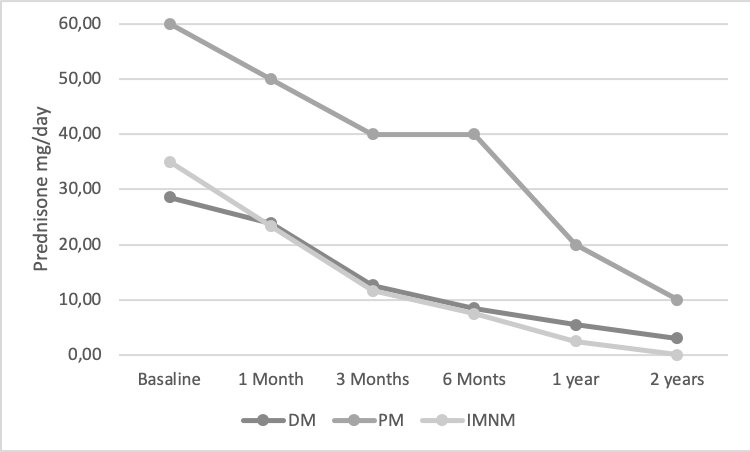
Treatment efficacy was studied based on a) clinical variables (muscle weakness and skin involvement), b) analytical variables (Creatin Kinase CK) and c) corticosteroid sparing effect. All these factors were measured at 1 month, 3 months, 6 months, 1 year and 2 years after starting IVIG treatment.
Muscle weakness was measured in 3 territories (upper limb, lower limb and neck flexor muscles) using the MRC (Medical Research Council Grading System) muscle strength scale.
The evolution of clinical parameters was classified into 3 categories: complete improvement (resolution), partial improvement and no improvement.
The IVIG treatment schedule used was 2g/kg spread over 2-5 days.
Results: A total of 37 patients with IIM were included, 29 females (78%) and 8 males (22%), with a mean age 47 ± 27 years. The most frequent group was DM (n=27; 73%), followed by IMNM (n=6; 16%), PM (n=3; 8%) and ASS (n=1; 3%).
Eight (30%) patients with DM received IVIG as induction therapy associated with standard treatment, while 19 (70.37%) patients received IVIG after boluses of methylprednisolone (n=10; 53%), prednisone 45 mg/day (n=19; 100%), or associated with other immunosuppressants (IS) (methotrexate, azathioprine, cyclophosphamide or mycophenolate). The main indication for IVIG in this group was muscle weakness (n=19; 70%) and skin involvement (n=8; 30%). The main indication for IVIG in MD was muscle weakness (n=19; 70%) and skin involvement (n=8; 30%).
All PM patients (n=3) had received previous treatment with Prednisone 60mg/day. The main indication was muscle weakness (n=3, 100%).
Of the 6 patients with IMNM, 5 (67%) received IVIG as induction therapy and 1 patient (33%) after Prednisone 60 mg/day. In all of them the indication was muscle weakness.
Only one patient with ASS received IVIG treatment, due to interstitial lung involvement and muscle weakness.
An improvement in muscle weakness (total and/or partial) (Figure 1), CK levels (Figure 2) and a sparing glucocorticoid effectof IVIGwas observed in all IIM subtypes.(Figure 3)
Conclusion: IVIGs are effective in all 3 subtypes of IIM studied.
To cite this abstract in AMA style:
Corrales Selaya C, Martínez-Lopez D, Prieto-Peña D, Benavides F, Martin-Varillas J, Blanco R. Intravenous Immunoglobulins in the Treatment of Idiopathic Inflammatory Myopathies in a Region of Northern Spain: Analysis by Subtypes [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/intravenous-immunoglobulins-in-the-treatment-of-idiopathic-inflammatory-myopathies-in-a-region-of-northern-spain-analysis-by-subtypes/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intravenous-immunoglobulins-in-the-treatment-of-idiopathic-inflammatory-myopathies-in-a-region-of-northern-spain-analysis-by-subtypes/