Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: The current standard of intraarticular therapy in patients with inflammatory arthritis (e.g. rheumatoid arthritis; RA) or inflammatory exacerbations of osteoarthritis (OA) is the injection of steroids, which can increase risk of infection, cartilage degenerations, and other well-known systemic side effects. A novel approach without such complications could be the activation of peripheral opioid receptors, e.g. by i.a. application of small, systemically inactive doses of morphine.
The aim of this placebo- and active drug controlled double blind trial was to investigate reduction of pain in knee arthritis patients following intraarticular (i.a.) injections of morphine 3 mg, a standard steroid (triamcinolone 40 mg), or placebo (Saline). The primary hypothesis was that i.a. morphine results in significantly lower pain scores than placebo, an efficacy comparable to standard i.a. steroid medication. The primary outcome parameter was reduction of the VAS pain at day 7.
Methods: Adult patients with active knee arthritis and a high level of pain (VAS pain ≥ 4) at baseline received a single dose of either morphine, or triamcinolone or placebo i.a.. Patients were monitored closely throughout a total of 2 visits over 2 weeks and documented pain in the morning and evening in a patient’s diary. Safety data was collected during the whole study period. P-values were calculated using two-sided T-tests.
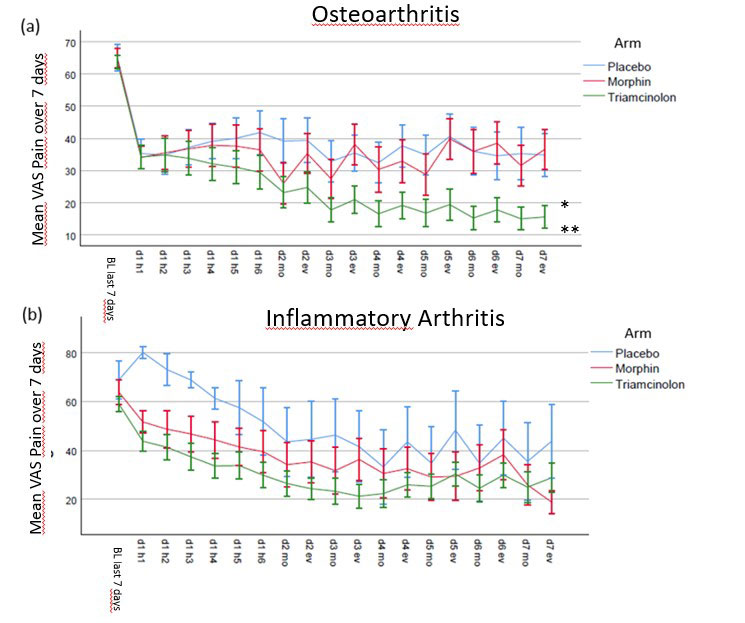
Results: 114 patients were screened, 93 were treated and 89 (96%) completed day 7. Of these n= 61 (66%) were diagnosed with OA and n= 32 (34%) with IA. 48 (52%) patients were female, mean age was 58.5 (SD 14 ys) and mean disease duration 6.7ys (median 2ys, range < 1y – 42ys, IQR < 1 – 10 ys). The mean VAS pain improvement at day 7 for morphine, triamcinolone and placebo was -22.8, -37.7, and -19.8 respectively. The difference was not significant (p=0.69) for placebo vs. morphine, but significant for placebo vs. triamcinolone and for triamcinolone vs. morphine (p=0.013 and p=0.006). The difference between the treatment groups in IA were not significant, while in OA triamcinolone was significantly superior when compared to morphine and to placebo (p= 0.007, p = 0.003), but not for morphine to placebo (p=n.s.). Mean improvements of the everyday pain documentation of both groups are shown in Figure 1. During the study period there were no serious adverse events and 45 adverse events, most of them were mild.
Conclusion: In this placebo and active controlled double blind trial a single dose of i.a. administered morphine did not lead to significant improvements in comparison to placebo and was inferior to triamcinolone at day 7. These data do not support the use of a small dosage of morphine intraarticularly in patients with knee arthritis.
To cite this abstract in AMA style:
Haibel H, Sieper J, Poddubnyy D, Rios-Rodriguez v, Proft F, Protopopov M, Rademacher J, Igel S, Martus P, Stein C. Intraarticular Morphine in Knee-arthritis – Results of a Randomized Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/intraarticular-morphine-in-knee-arthritis-results-of-a-randomized-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intraarticular-morphine-in-knee-arthritis-results-of-a-randomized-placebo-controlled-trial/