Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) occurs in about 50% of patients with systemic sclerosis (SSc). The aims of this study were to assess the prevalence of ILD and describe its clinical and radiological characteristics in very early SSc (veSSc), and to investigate ILD development during follow-up.
Methods: In this longitudinal cohort study, we included patients with veSSc, defined as Raynaud’s phenomenon (RP) and/or at least one of either puffy fingers, antinuclear antibodies (ANA), or abnormal capillaroscopy, not fulfilling the 2013 ACR/EULAR classification criteria for SSc at baseline. High-resolution computer tomography of the thorax (HRCT) is routinely done in veSSc patients in our center. Only patients with available HRCT data were included. An expert thoracic radiologist reevaluated all HRCTs blindly, according to a predefined protocol describing radiologic abnormalities, pattern of ILD and radiologic progression at follow-up. We analyzed associations with relevant clinical parameters at baseline using Fisher`s exact test. Longitudinally, we assessed ILD onset or progression, defined by HRCT or worsening of the pulmonary function tests (PFT) (absolute decline in forced vital capacity (FVC) >5% or diffusing capacity for carbon monoxide (DLCO) >10%; relative decline in FVC ≥ 10% or between 5-9% and DLCO ≥15%). Treatment data were collected from the electronic records.
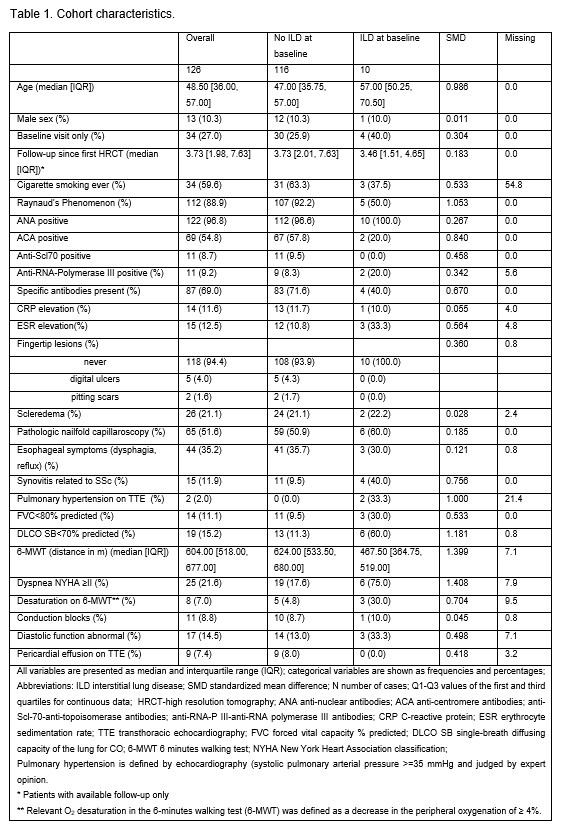
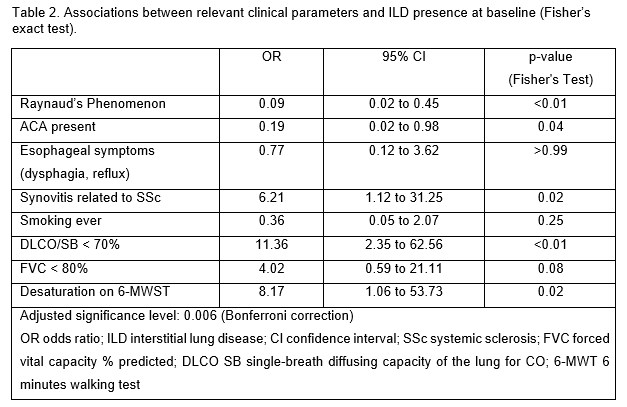
Results: Baseline characteristics of the 126 included patients are shown in Table 1. Among them, 15 patients (11.9%) patients had ILD, 10 (7.9%) at baseline. Non-specific interstitial pneumonia (NSIP) was identified in 3/10 patients, probable usual interstitial pneumonia (UIP) in 2/10, whereas reticular changes with no clear pattern were described in the remaining patients. The extent of ILD was < 10% in 5/10 patients, 10-20% in 4/10 and >20% involvement in 1/10. More than half of the patients were symptomatic (6/8 had dyspnea NYHA class ≥ II). There was a strong association between ILD at baseline and reduced DLCO < 70% (p < 0.001), but not with reduced FVC (Table 2). Patients with ILD at baseline had less frequent RP, more often arthritis and desaturation on the 6-MWT (Table 2). During a median follow-up of 3.5 years, 5 patients developed new ILD and 2 progressed, 1 on both HRCT ( >20%), PFT and increase of NYHA dyspnea grade II to III, and 1 only in PFT. NSIP was diagnosed in 2/5 new cases, while the remaining ones had no typical radiologic pattern. At diagnosis visit, the extent of the reticular changes was < 10% in all 5 cases.
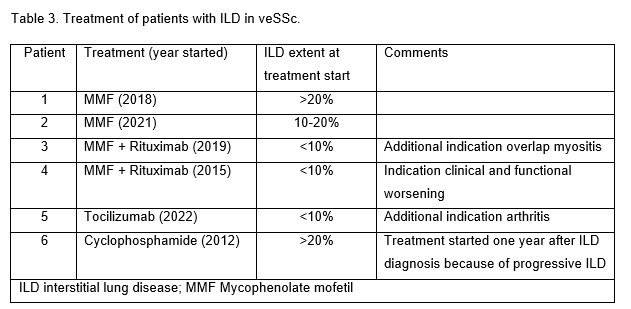
Disease-modifying treatment was started in 5/15 patients at the visit when ILD was diagnosed, another 1 patient received Cyclophosphamide at the first follow-up visit (Table 3), as recommended by the treating physician.
Conclusion: ILD occurs even in veSSc, albeit at a low prevalence of 8%. Half of the patients with veSSc-ILD had lung involvement >10%, more than half showed low DLCO, relevant dyspnea and a third required ILD therapy. A reduced DLCO < 70% was strongly associated with ILD at baseline. New onset ILD or progression was observed at follow-up. This highlights the need for screening for ILD in veSSc, including both HRCT and PFT, to allow early diagnosis and stratification of affected patients.
To cite this abstract in AMA style:
Muraru S, Blüthgen C, Bruni C, Mihai C, Hoffmann-Vold A, Elhai M, Becker M, Frauenfelder T, Distler O, Dobrota R. Interstitial Lung Disease in Very Early Systemic Sclerosis – a Clinical and Radiological Characterization [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/interstitial-lung-disease-in-very-early-systemic-sclerosis-a-clinical-and-radiological-characterization/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interstitial-lung-disease-in-very-early-systemic-sclerosis-a-clinical-and-radiological-characterization/