Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Consensus-derived treat-to-target (T2T) goals for childhood-onset SLE (cSLE), including clinical remission on low dose steroids (cCR), have been endorsed by the Paediatric Rheumatology European Society (PReS). This study evaluated whether components of cCR could either be removed or transformed to create modified definitions that provide better protection against high disease activity, as compared to the original consensus-derived cCR.
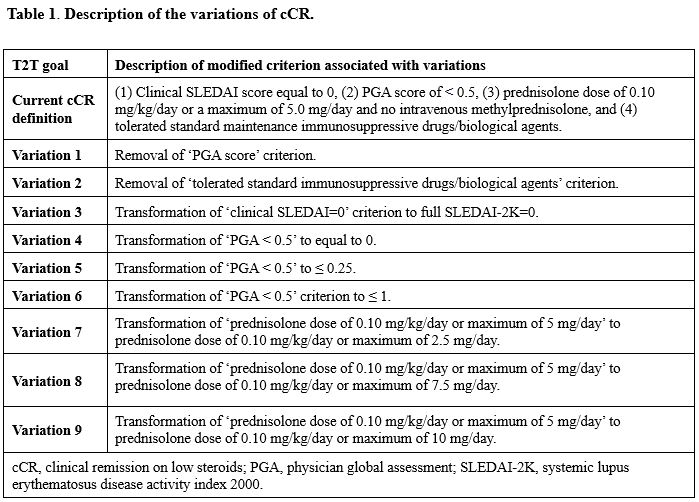
Methods: Patients from UK JSLE Cohort Study and the CARRA Registry who were < 18 years at diagnosis and met ³4 ACR/SLICC criteria were included. The components of cCR were either eliminated or transformed as described in Table 1, with 9 variations explored. Prentice-Williams-Peterson (PWP) gap-time models assessed the impact of the new cCR target definitions on high disease activity (SLEDAI-2K ≥10), with the resulting hazard ratios (HRs) compared between original and varied cCR definitions using Student’s t-tests. Target attainability was compared between the original cCR and its corresponding variations using Wilcoxon tests.
Results: Data were available from 1,566 cSLE patients (11,965 visits; UK JSLE: n=492, 5,923 visits; CARRA: n=1,074, 6,042 visits). Achieving the original cCR (HR 0.24 [CI 0.18, 0.33]) reduced the hazards of subsequent high disease activity by 76% (p < 0.001, Table 2). Removing the physician global assessment (PGA) criterion (HR 0.31 [CI 0.25, 0.39]) or the criterion for stable immunosuppression (HR 0.27 [CI 0.21, 0.36]) statistically significantly worsened the risk of high disease activity, compared to the original definition (all p < 0.001, Table 2). Transformation of the clinical SLEDAI=0 criterion to incorporate full SLEDAI-2K=0 (Transformation 3) significantly improved the hazard of high disease activity compared to original cCR (p < 0.001, Table 2). However, this transformation significantly increased time-to-target attainment (Original cCR: 18 months vs. Transformation 3: 20 months) and significantly reduced the time spent in target (Original cCR: 11.3 months vs. Transformation 3: 7.4 months).
Conclusion: These analyses demonstrate that PGA and stable immunosuppression are both essential to defining clinical remission. Although transformation of the clinical SLEDAI criterion to incorporate the full SLEDAI-2K reduced the hazards of high disease activity, this made the definition of remission significantly less attainable, suggesting that the original definition, derived from the adult definition, is optimal for use in cSLE. Further research should evaluate the consensus and varied cCR definitions in their ability to predict and prevent lupus-related damage.Disclaimer: This study utilized data collected in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The views expressed are the authors’ and do not necessarily represent the view of CARRA.Acknowledgements: This work could not have been accomplished without the aid of the NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the Arthritis Foundation (AF), and the Centers for Disease Control and Prevention (CDC). We would also like to thank all participants and hospital sites that recruited patients.
To cite this abstract in AMA style:
Sarker C, Cooper J, Smitherman E, Alves F, Belot A, Beresford M, Jorgensen A, Smith E, Lewandowski L, Sadun R. International Assessment of cSLE Clinical Remission (cCR) Criteria in Childhood Lupus: Sensitivity Analyses from the UK JSLE Cohort and the CARRA Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/international-assessment-of-csle-clinical-remission-ccr-criteria-in-childhood-lupus-sensitivity-analyses-from-the-uk-jsle-cohort-and-the-carra-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/international-assessment-of-csle-clinical-remission-ccr-criteria-in-childhood-lupus-sensitivity-analyses-from-the-uk-jsle-cohort-and-the-carra-registry/

.jpg)