Session Information
Date: Saturday, November 16, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The presence of systemic inflammation during gout flares is well characterized. While initial research suggests inflammation persists in intercritical gout, these reports have come predominantly from small observational cohorts with a limited number of biomarkers. The objective of this study was to examine whether measures of systemic inflammation differ between gout patients during the intercritical period and age-matched controls in a large, well-phenotyped sample of participants from a randomized controlled trial (RCT).
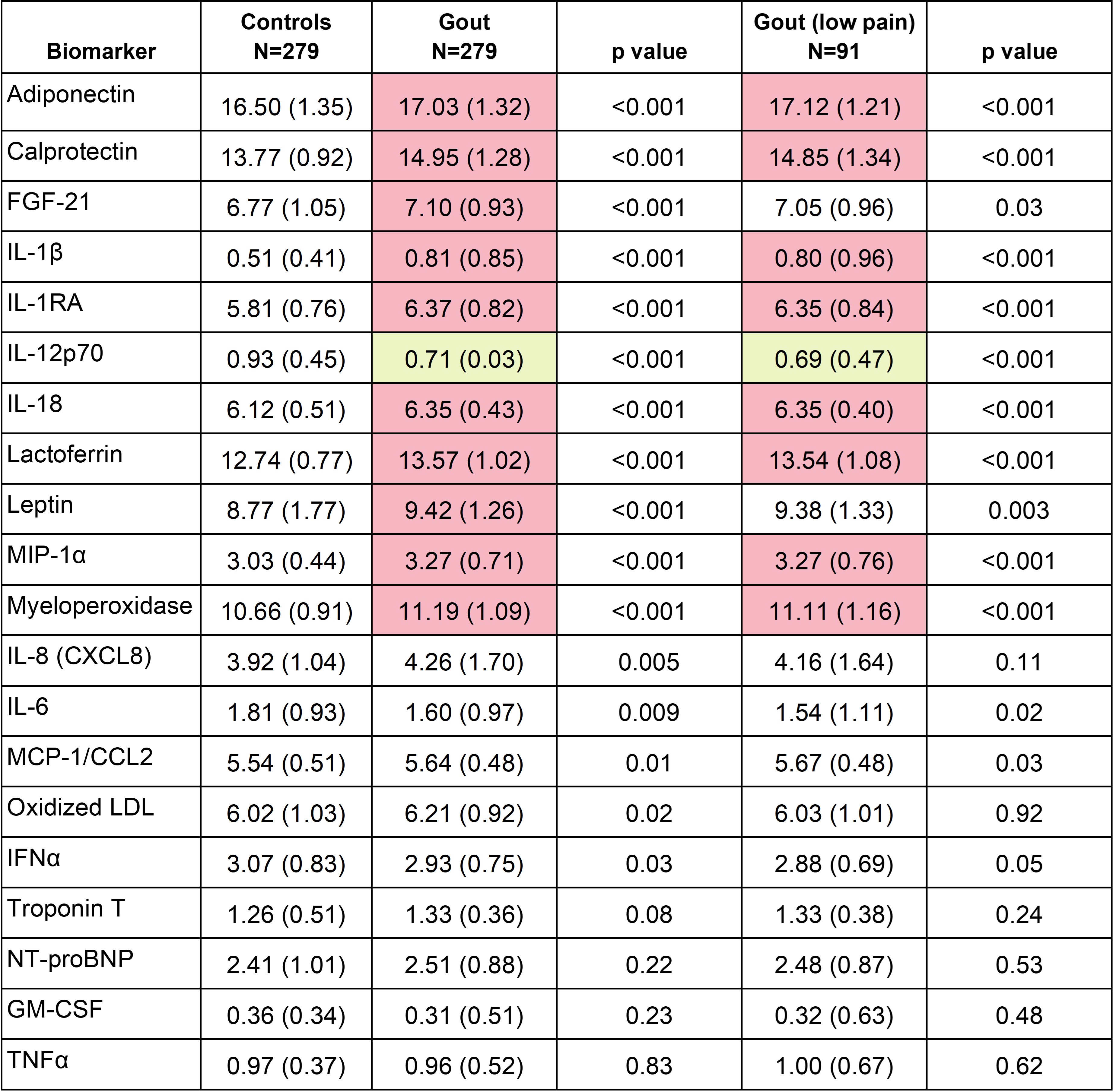
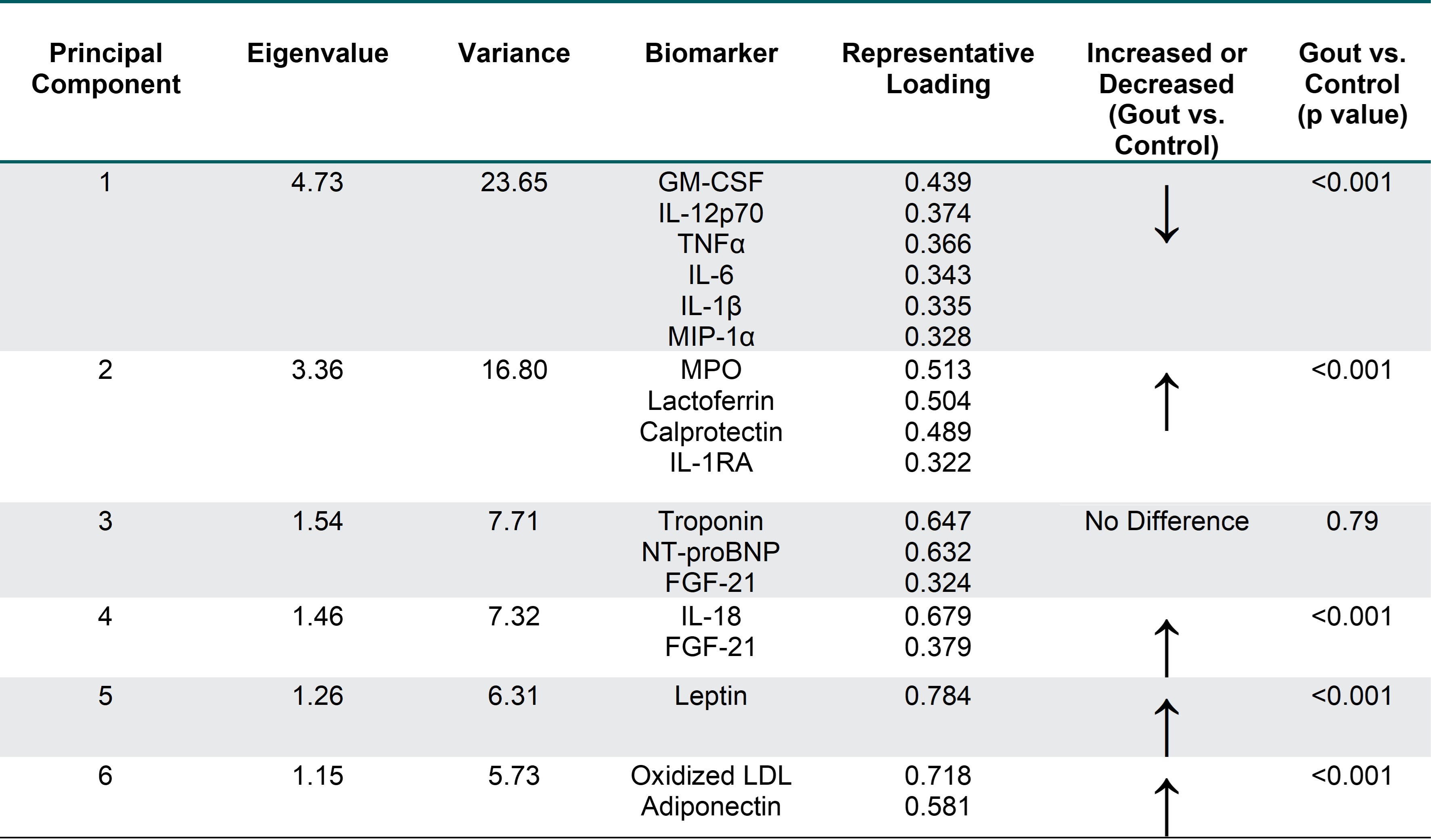
Methods: This cross-sectional study leveraged baseline serum samples (prior to therapy) from a subcohort of gout patients (n=282) enrolled in a large RCT comparing urate-lowering therapies following a treat-to-target approach. Comparisons were made using serum from age-matched controls (n=282) without gout (oversampling men to more closely reflect gout demographics). A panel of 20 biomarkers, selected based on published associations with gout or gout-related therapies, was measured using commercial assays with values normalized for analyses. Values were compared between groups using Student’s t-tests and Bonferroni corrected p-values (p < 0.0025). In secondary analyses designed to exclude gout patients possibly experiencing flare, gout participants reporting moderate/high pain (on EuroQol 5 dimension [EQ5D] score) were excluded. Principal component analysis (PCA) was then used to identify unique biomarker profiles using pooled data from all gout cases and controls. A multivariable logistic regression model was used to examine associations between all 6 principal component (PC) scores and gout status.
Results: Participants with gout (n=282) had a mean age of 70.0 years, a mean BMI of 33.1 kg/m2, were predominantly male (96.4%), and reported White race (75.2%). The mean sUA was 8.51 mg/dl. Controls shared similar characteristics with the exception of a lower frequency of males (80.0%) and lower mean BMI (30.6 kg/m2). Eleven of the 20 biomarkers differed significantly by disease status, with 10 being higher in gout (Table 1). Biomarker values observed overall in gout were similar in the subgroup reporting low/no pain. Nine of 11 biomarkers retained significance in secondary analyses excluding gout patients reporting moderate/high pain. PCA yielded 6 PCs (Eigenvalues >1) that cumulatively explained 67.5% of variance (Table 2). PCs 1, 2, and 4 were characterized by inflammatory cytokines/chemokines, while PCs 5 and 6 were characterized by adipokines. Each of these PCs were associated with gout. PC 3 was comprised of cardiac biomarkers and was not associated with gout.
Conclusion: Compared to controls, patients with intercritical gout exhibit evidence of subclinical inflammation characterized by alterations in multiple circulating pro-inflammatory cytokines. In addition to highlighting gout as a chronic systemic disease, these results suggest that subclinical inflammation during the intercritical period could contribute to gout outcomes, including the high burden of comorbidity that characterizes this condition. An important question is then whether this can be meaningfully modified with effective gout management.
Values significantly higher in gout (p<0.0025) shaded in red; values significantly lower in gout shaded in yellow. Measurements performed via sandwich enzyme-linked immunosorbent assay (oxidized low-density lipoprotein) or Mesa Scale Discovery assay (all others).
Abbreviations: fibroblast growth factor (FGF), interleukin (IL), IL_1RA (interleukin_1 receptor antagonist), macrophage inflammatory protein (MIP), monocyte chemoattractant protein (MCP), low density lipoprotein (LDL), interferon (IFN), N-terminal pro-B-type natriuretic peptide (NT-proBNP), granulocyte-monocyte stimulating factor (GM-CSF), tumor necrosis factor (TNF)
Abbreviations: granulocyte-monocyte stimulating factor (GM-CSF), interleukin (IL), macrophage inflammatory protein (MIP), tumor necrosis factor (TNF), IL_1RA (interleukin_1 receptor antagonist), N-terminal pro-B-type natriuretic peptide (NT-proBNP), MPO, myeloperoxidase, fibroblast growth factor (FGF), low density lipoprotein (LDL), interferon (IFN), monocyte chemoattractant protein (MCP); only analytes shown with absolute loading scores >0.3 (no IL-8, IFNα, or MCP_1); p-value generated from multivariable logistic regression that included the 6 PC scores as independent variables and case status as the dependent variable
To cite this abstract in AMA style:
Ourada T, Wheeler A, Duryee M, England B, Reynolds R, O'Dell J, Newcomb J, Pillinger M, Terkeltaub R, Ferguson R, Brophy M, Merriman T, Mikuls T. Intercritical Gout Represents a Systemic Inflammatory State [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/intercritical-gout-represents-a-systemic-inflammatory-state/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intercritical-gout-represents-a-systemic-inflammatory-state/