Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: ACPA detection assays are often used for RA diagnosis due to their high specificity ( >90%). However, current ACPA assays (e.g., anti-CCP2 ELISA) have far lower sensitivity (30–60%), and thus patients can still be clinically diagnosed with RA while testing negative for ACPA (or otherwise known as the “ACPA-negative” RA subgroup). This can make the diagnosis of RA quite challenging for early-stage RA patients, resulting in delays in starting therapeutic interventions. In addition, ACPA-negative RA patients are about 40% of the RA patient population and can show different disease course compared to ACPA-positive patients. To better understand the biomolecular characteristics of ACPA-negative RA given its clinical significance, we performed an integrative multi-omic phenotyping analysis on blood (plasma) samples from RA patients of two different subgroups (i.e., ACPA-negative, ACPA-positive) and healthy controls. Our aims were to identify features specific to ACPA-negative RA and potential biomarkers for ACPA-negative RA diagnostics.
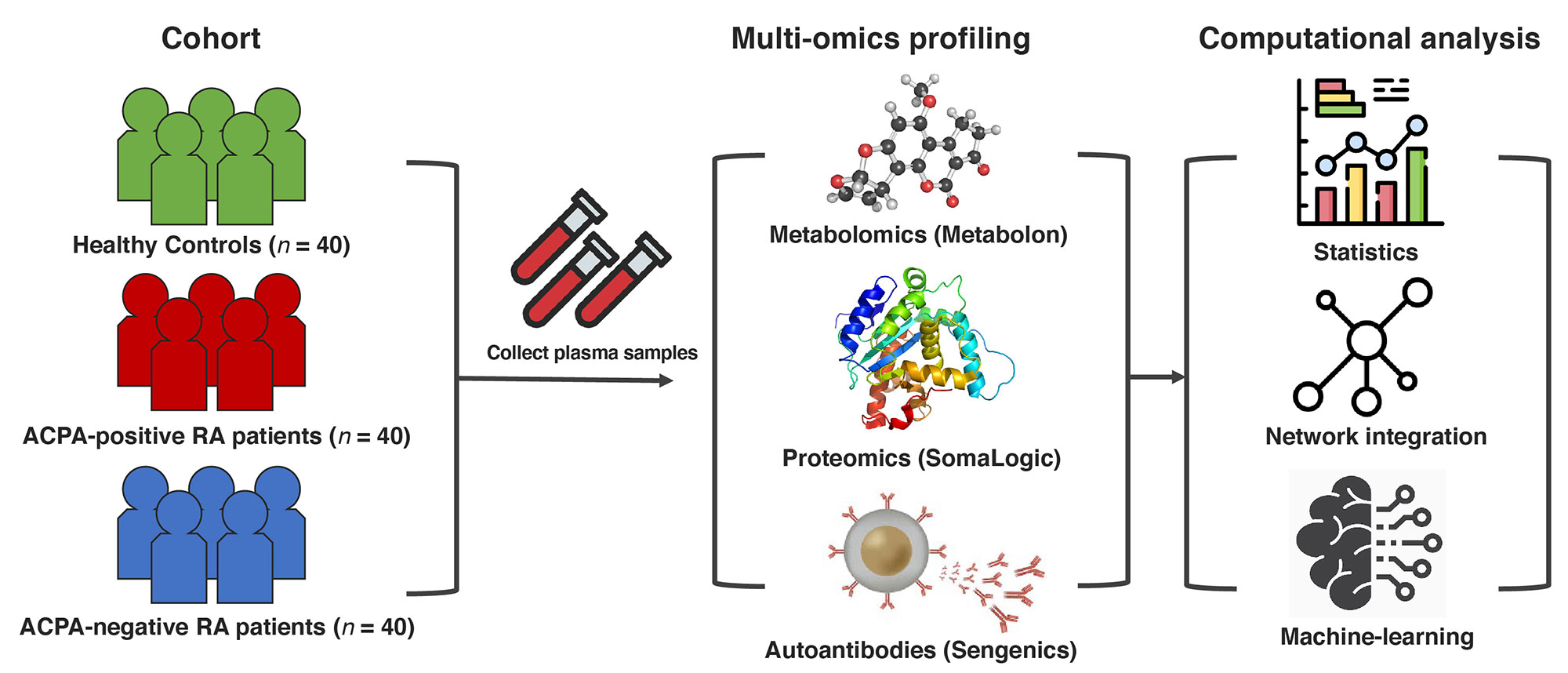
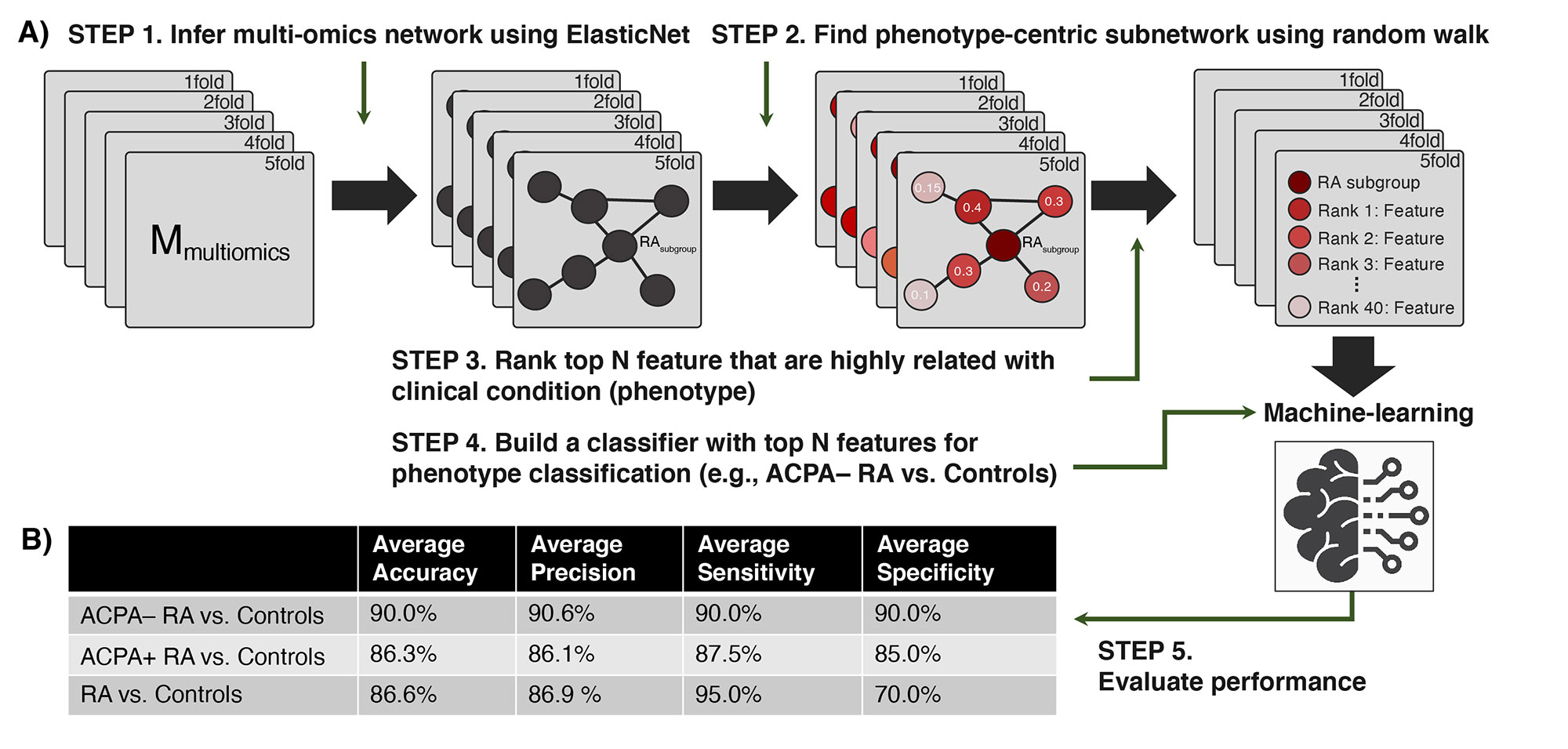
Methods: Global proteomic, metabolomic, and autoantibody profiling were performed on plasma samples from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40) (Fig. 1). A linear regression model was used to identify multi-omics features associated with ACPA-negative RA. ElasticNet was used to infer a multiplex network (from the multi-omics data), which characterizes associations among multi-omic features and clinical conditions in a single analytical framework (Fig. 2A, Steps 1–2). A machine-learning strategy using a network-based feature selection scheme was developed for RA subgroup classification (Fig. 2A, Steps 3–5).
Results: We identified various plasma proteins, metabolites, or autoantibodies associated with RA subgroups. In particular, compared to healthy controls, we identified 23 upregulated and 49 downregulated plasma proteins in ACPA-negative RA; and 25 upregulated and 32 downregulated plasma proteins in ACPA-positive RA. Proteins that were differentially abundant in ACPA-negative RA were found to be enriched in innate immune response and metabolic processes, while proteins that were differentially abundant in ACPA-positive RA were found to be enriched in chemotaxis- and cell migration-related functions. Interestingly, the identified differentially abundant metabolites and autoantibodies were found to support our proteomics results. Finally, our network-based machine-learning classification scheme was able to distinguish ACPA-negative RA patients from healthy controls with 90.0% accuracy, and classify RA patients (of both subgroups) and healthy controls with 86.6% accuracy (Fig. 2B).
Conclusion: We demonstrate the first simultaneous proteomic, metabolomic, and autoantibody phenotyping in the blood of patients with ACPA-negative RA. Our computational analysis identifies multi-omics features that may explain the similarity and differences in biomolecular processes between the ACPA-negative and ACPA-positive subgroups of RA; and suggests potential biomarkers with clinical applicability in blood-based RA diagnostics.
To cite this abstract in AMA style:
Hur B, Cunningham K, Davis J, Sung J. Integrative Multi-omic Phenotyping in Blood Identifies Molecular Signatures and Candidate Biomarkers of ACPA-negative Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/integrative-multi-omic-phenotyping-in-blood-identifies-molecular-signatures-and-candidate-biomarkers-of-acpa-negative-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrative-multi-omic-phenotyping-in-blood-identifies-molecular-signatures-and-candidate-biomarkers-of-acpa-negative-rheumatoid-arthritis/