Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Lupus nephritis (LN) is driven by a heterogeneous population of renal macrophages. We have previously reported that the renal macrophage subpopulations are similar in mouse and human LN. In both species, disease-associated sub-populations of infiltrating (classical 2) and resident macrophages correlate with higher LN activity and chronicity scores respectively and express genes associated with Scar Associated Macrophages (SAMs) including CTSB, SPP1, FABP5, TREM2, and CD63. However, the function of these cells in LN kidneys are still unknown. Here, we elucidate the phagocytic capacity and effects of these disease-associated macrophage subsets on fibroblast cells in-vitro.
Methods: We used flow cytometry to identify macrophage subsets in nephritic kidneys of two commonly employed lupus mouse models, NZB/W and SLE1.Yaa. Phagocytic capacity was measured by flow cytometry after cell exposure to immune complexes, apoptotic cells and Latex beads ex vivo. Because infiltrating SAMs constitute only a small population of renal cells, we developed an in-vitro culture system by polarizing SLE1.Yaa bone marrow-derived macrophages (BMDMs) with GM-CSF and IL-17A, resulting in induced SAMs (iSAMs) and confirmed their phenotype with flow cytometry and RNA sequencing. The ability of iSAM-derived supernatants to promote wound repair was assessed through fibroblast migration and fibroblast density in a scratch wound assay. Similar experiments were performed using resident macrophages isolated from either pre-nephritic or nephritic LN kidneys.
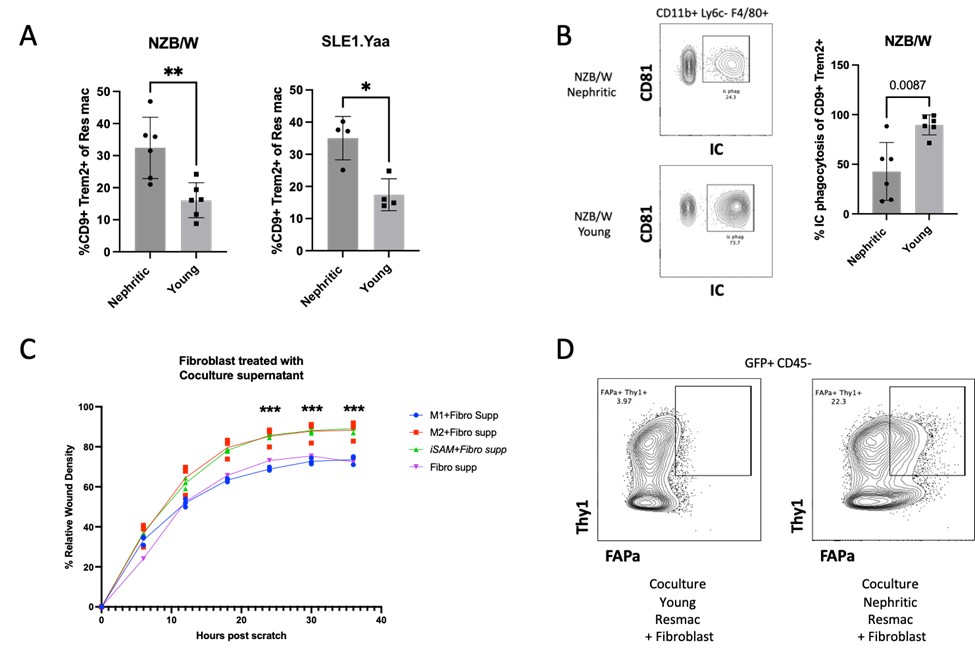
Results: Nephritic mice had a higher frequency and number of both infiltrating and resident macrophage (Fig 1A) CD9+ Trem2+ SAMs compared to non-nephritic controls. Nephritic resident macrophages had a profound defect in phagocytosis of immune complexes (Fig 1B) and apoptotic cells but not of latex beads. Phagocytosis was not defective in renal classical 1 or classical 2 macrophages from nephritic mice. The in-vitro-derived GM-CSF and IL-17A and M2 polarized BMDM expressed significantly more CD9 and Trem2 compared to M0 and M1 macrophages. When the scratch wound was exposed to the iSAM-fibroblast co-culture supernatant, fibroblast migration, and wound density were increased in both GM-CSF and IL-17A and M2 polarized BMDM compared to M0 and M1 (Fig 1C). Nephritic resident macrophages enhanced fibroblast density within the wound area compared to pre-nephritic resident macrophages and induced an activated FAP+ fibroblast phenotype (Fig 1D).
Conclusion: Infiltrating and resident macrophages that correlate with lupus nephritis disease severity include SAMs. Resident macrophages but not infiltrating macrophages from nephritic mice display a receptor-mediated phagocytosis defect. When SAMs are derived in-vitro with IL-17a and GM-CSF their supernatant enhances fibroblast migration and density within the wound. Similarly, resident macrophages from nephritic mice display a functional pro-fibrotic phenotype compared with those from pre-nephritic mice. These studies suggest that therapeutic targeting of SAMs may attenuate fibrosis in LN.
To cite this abstract in AMA style:
Raparia C, Hoover P, Arazi A, Hacohen N, Davidson A. Infiltrating and Resident Macrophages in Lupus Nephritis: Scar Associated Macrophage Phagocytic Dysfunction and Fibroblast Activation [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/infiltrating-and-resident-macrophages-in-lupus-nephritis-scar-associated-macrophage-phagocytic-dysfunction-and-fibroblast-activation/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infiltrating-and-resident-macrophages-in-lupus-nephritis-scar-associated-macrophage-phagocytic-dysfunction-and-fibroblast-activation/