Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Antiphospholipid syndrome (APS) is an acquired autoimmune thrombophilia characterized by circulating antiphospholipid antibodies (aPL). While the association between aPL and infection has long been recognized, the factors that trigger the production of aPL during infection are largely unknown, and the clinical significance of those infection-associated aPL continues to be debated. Here, we endeavored to comprehensively evaluate the presence of aPL in a large cohort of critically ill sepsis patients and non-sepsis intensive care unit controls. We further investigated whether purified IgG fraction from APS patients with high anti-phosphatidylserine/prothrombin (aPS/PT) IgG can modulate neutrophil activation and neutrophil extracellular traps (NETs) formation in the mouse sepsis model.
Methods: Plasma from 231 critically ill sepsis and 94 non-sepsis patients were evaluated for eight different types of aPL: anticardiolipin (aCL) IgG/IgM/IgA, anti-beta-2 glycoprotein I (aβ2GPI) IgG/IgM/IgA, aPS/PT IgG/IgM with ELISA kits (Werfen), Human E-Selectin/CD62E and BAFF/BLyS/TNFSF13B DuoSet ELISA (R&D Systems). An intranasal Klebsiella pneumonia-induced mouse sepsis model was used to evaluate the potential role of patient-derived aPL in sepsis outcomes.
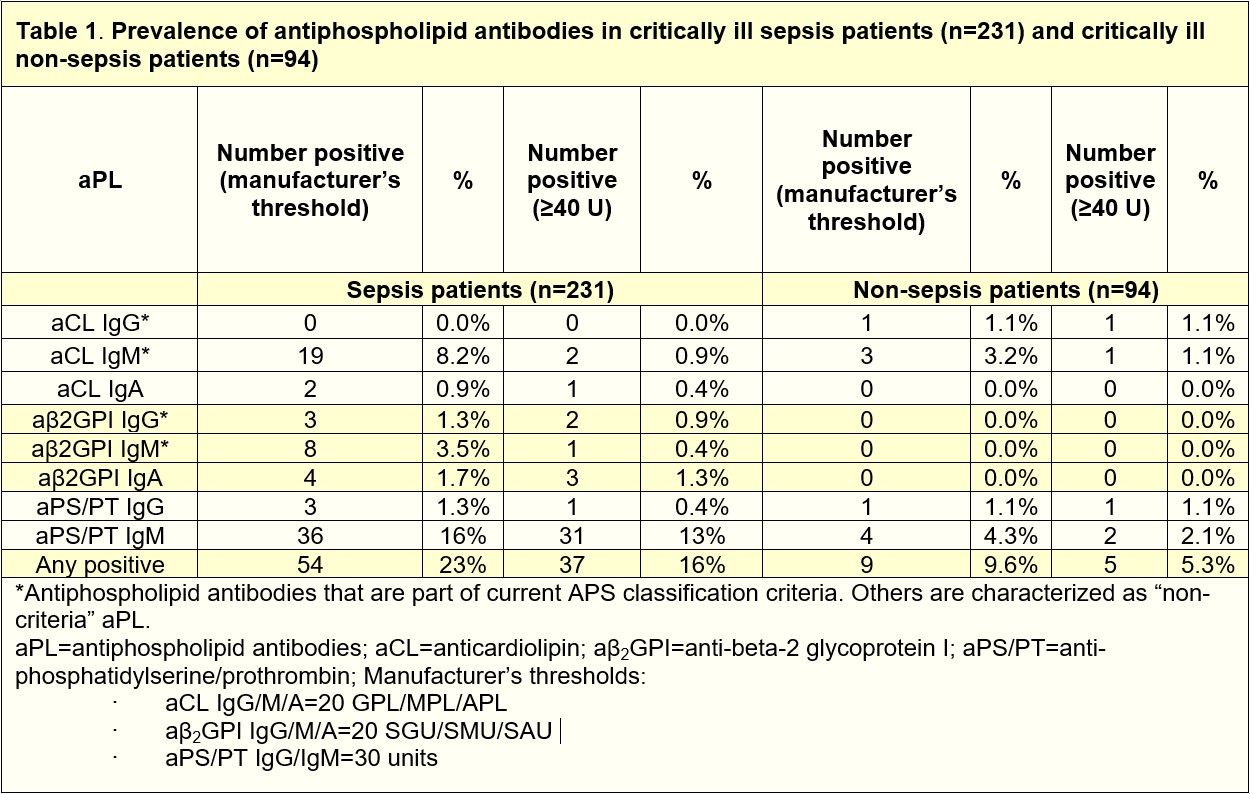
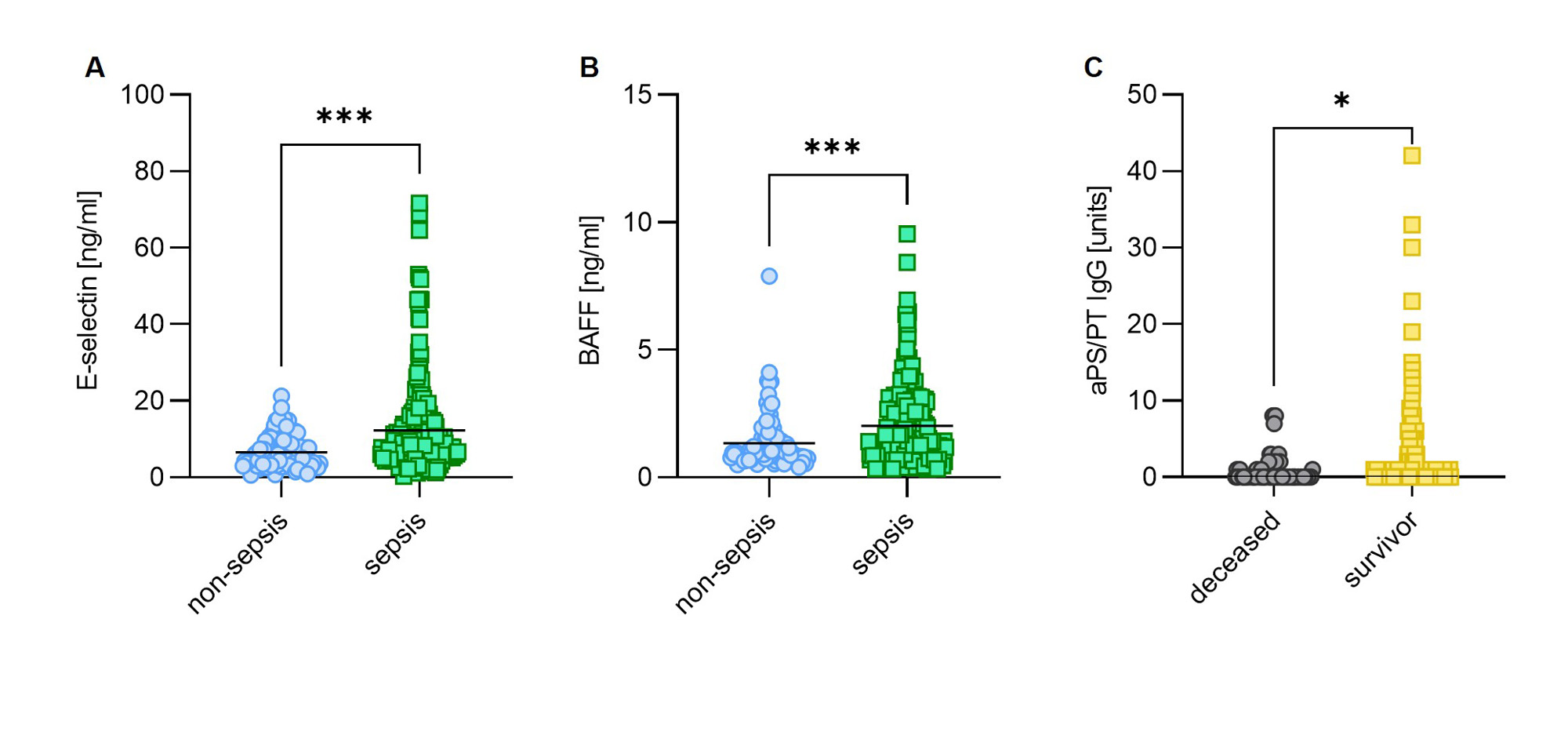
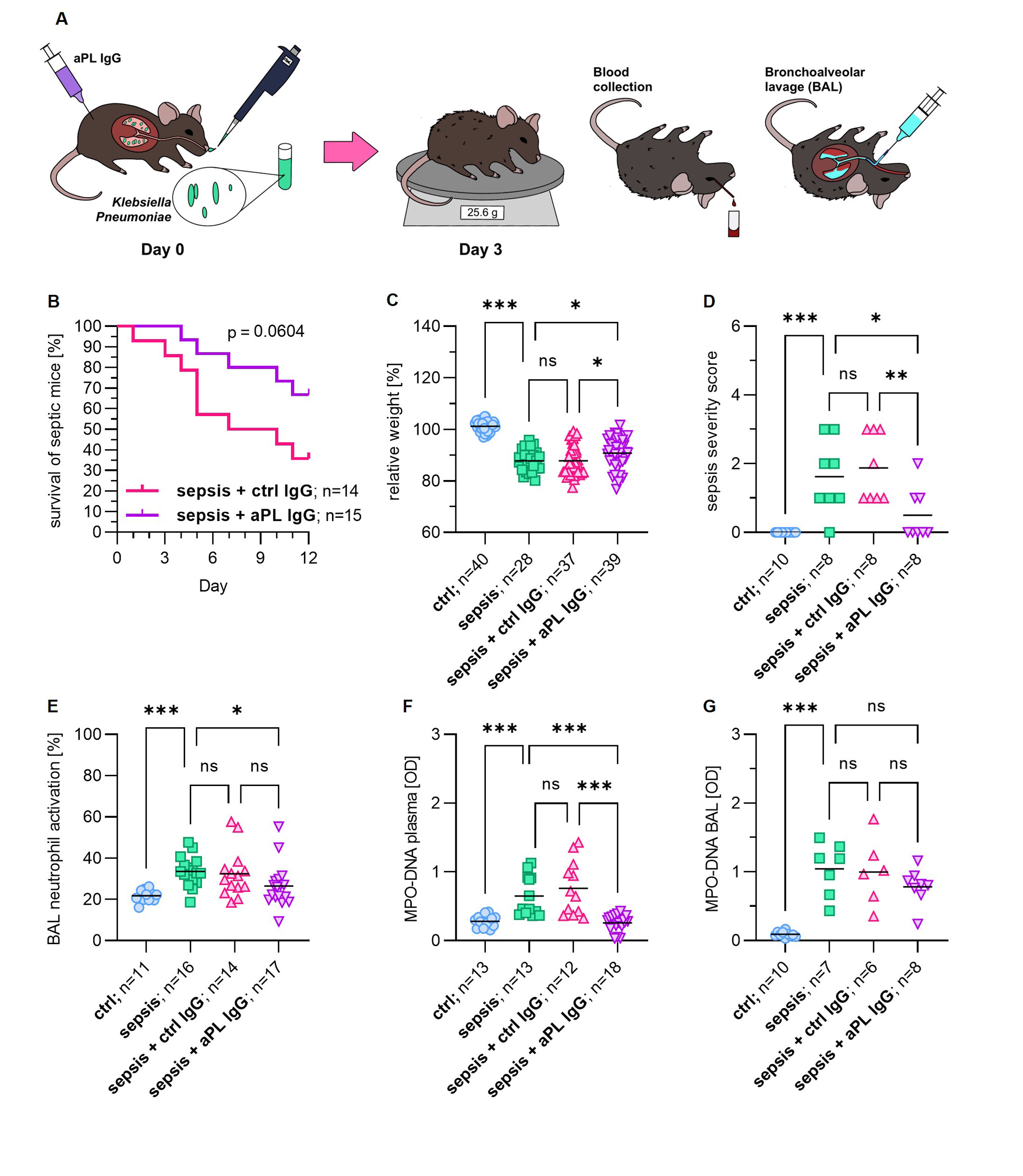
Results: We found that 23% of critically ill sepsis patients had at least 1 positive aPL, as compared with 9.6% of non-sepsis patients. The most prevalent aPL found among sepsis patients was aPS/PT IgM (16%), followed by aCL IgM (8.2%), and aβ2GPI IgM (3.5%). When considering only aPL at moderate-to-high titer, 16% of critically ill sepsis patients had at least 1 positive aPL compared to 5.3% of non-sepsis patients (Table 1). High aPL levels, such as aPS/PT IgM demonstrated a positive correlation with soluble E-selectin (r=0.21, p=0.039), a marker of endothelial cell activation. Higher levels of E-selectin were found in critically ill sepsis patients compared to non-sepsis patients (Fig 1A). To evaluate a potential trigger of infection-associated aPL, we measured BAFF, a B cell-activating factor that promotes the differentiation of autoantibody-secreting cells. We found markedly elevated BAFF among critically ill sepsis patients (Fig 1B). Interestingly, patients who survived sepsis were more likely to have elevated aPS/PT IgG as compared to those who did not (Fig 1C). To evaluate the extent to which aPL may improve sepsis outcomes, we set up an intranasal Klebsiella pneumoniae mouse sepsis model (Fig 2A). Our data suggest that the administration of IgG purified from patients with high-titer anti-PS/PT antibodies to septic mice led to a strong trend toward improved survival while ameliorating infection-associated weight loss and sepsis severity (Fig 2B-D). Interestingly, aPL treatment also attenuated in situ neutrophil activation and NET formation, which are well-known orchestrators of sepsis hyper-inflammation (Fig 2E-G).
Conclusion: Our study suggests that many critically ill sepsis patients can develop aPL, potentially driven by heightened B-cell stimulating signals. While further mechanistic studies are warranted, some aPL may contribute to improved sepsis survival.
To cite this abstract in AMA style:
Kmetova K, Chong E, Somanathapura N, Navaz S, Kluge L, Yalavarthi S, Knight J, Maile M, Zuo Y. Infection-Associated Antiphospholipid Antibodies and Their Potential Role in Sepsis Outcomes [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/infection-associated-antiphospholipid-antibodies-and-their-potential-role-in-sepsis-outcomes/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infection-associated-antiphospholipid-antibodies-and-their-potential-role-in-sepsis-outcomes/