Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In the COMET study, etanercept (ETN) plus methotrexate (MTX) therapy in patients with early rheumatoid arthritis (RA) yielded high clinical remission rates,1 but whether remission can be maintained after dose reduction or withdrawal (biologic-free) is unknown. The PRIZE trial is an ongoing, prospective,121-wk, 3-period study to evaluate the efficacy of ETN/MTX as first-line therapy in patients with early, active moderate-to-severe RA and to assess whether efficacy can be maintained with reduced-dose or biologic-free therapy (Period 2) or drug free (Period 3). The main objective of Period 1, reported here, was to achieve DAS28 remission at wks 39 and 52 in patients treated with ETN 50 mg QW plus MTX (ETN50/MTX); responders (DAS28 ≤3.2 at wk 39 + DAS28 <2.6 at wk 52) qualified for Period 2 enrollment.
Methods: MTX- and biologic-naïve RA patients (symptom onset ≤12 mo from enrollment; DAS28 >3.2) received ETN50/MTX for 52 wks. At the discretion of the investigator, the initial 10 mg/wk MTX dose was titrated up to a maximum of 25 mg/wk to achieve remission. Patients not achieving low disease activity (LDA; DAS28 ≤3.2) received corticosteroid boosts at wks 13 and/or 26; those not achieving LDA at wk 39 (non-responders) were withdrawn from the study. In addition to DAS28 and SDAI remission and LDA, other standard clinical outcomes were assessed. Efficacy and safety analyses were conducted in all patients who received ≥1 ETN/MTX dose (mITT).
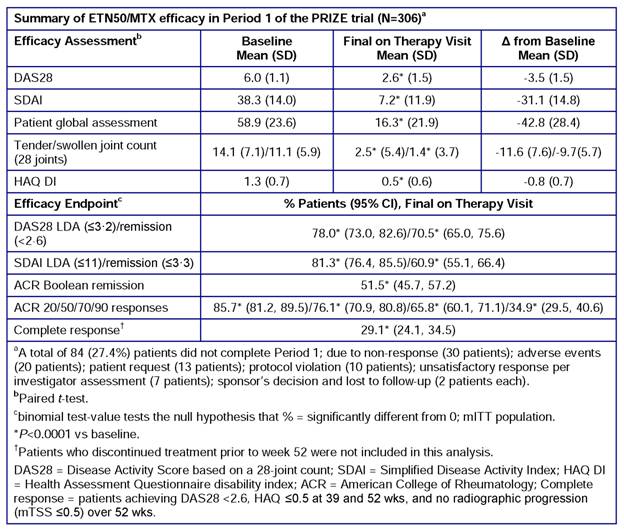
Results: A total of 306 patients (female, 70%; Caucasian, 94%; mean age, 50 y; disease duration from symptom onset, 6.5 mo) were enrolled; 222 (72.6%) completed Period 1 (reasons for not completing Period 1 are listed in the table). No patient was excluded from the mITT population. Efficacy results are summarized in the table. Significant changes from baseline were observed in all clinical assessments and endpoints (P<0.0001). LDA was achieved in >75% of patients and DAS28 and SDAI remission in >60% of patients. Twenty-nine percent of patients achieved DAS28 <2.6 + HAQ ≤0.5 + no radiographic progression. The most common treatment-emergent AEs were nausea and nasopharyngitis (13%, each). No unexpected safety or tolerability findings were reported.
Conclusion: In Period 1 of the PRIZE trial, the majority of patients with early, moderate-to-severe RA who received ETN50/MTX for 52 wks achieved remission. ETN/MTX was well tolerated, with no unexpected safety issues. Forthcoming results from Periods 2 and 3 will address whether this initial response to combination therapy will be maintained with ETN dose reduction, biologic-free, or drug-free.
Reference: 1.Emery P, et al. Lancet 2008; 372: 375–82.
Disclosure:
P. Emery,
Pfizer Inc,
2,
Pfizer Inc,
5;
M. Hamoudeh,
Pfizer Inc,
2,
Pfizer Inc; ,
5;
O. FitzGerald,
Pfizer Inc,
2,
Pfizer Inc,
8;
B. Combe,
Pfizer Inc,
5,
Pfizer Inc,
8;
S. Gaylord,
Pfizer Inc,
1,
Pfizer Inc,
3;
T. Williams,
Pfizer Inc,
1,
Pfizer Inc,
3;
J. Bukowski,
Pfizer Inc,
5;
R. Pedersen,
Pfizer Inc,
1,
Pfizer Inc,
3;
A. S. Koenig,
Pfizer Inc,
1,
Pfizer Inc,
3;
B. Vlahos,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/induction-of-remission-in-patients-with-up-to-12-months-of-moderate-to-severe-rheumatoid-arthritis-symptoms-treated-with-etanercept-plus-methotrexate-over-52-weeks/