Session Information
Date: Friday, November 6, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Multimorbidity
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) with RA have an increased risk of some malignancies compared with the general population, and this can vary by region/race.1,2 Epidemiologic data on the incidence of malignancies in Japanese pts with RA are limited. There are also limited data reporting the impact of biologic (b)DMARDs and targeted synthetic (ts)DMARDs, such as JAK inhibitors, on the incidence of malignancies in Japanese pts with RA. The National Database of Rheumatic Diseases in Japan (NinJa) is one of the largest RA registries in Japan. This study evaluated the incidence of malignancies in Japanese pts with RA using NinJa registry data.
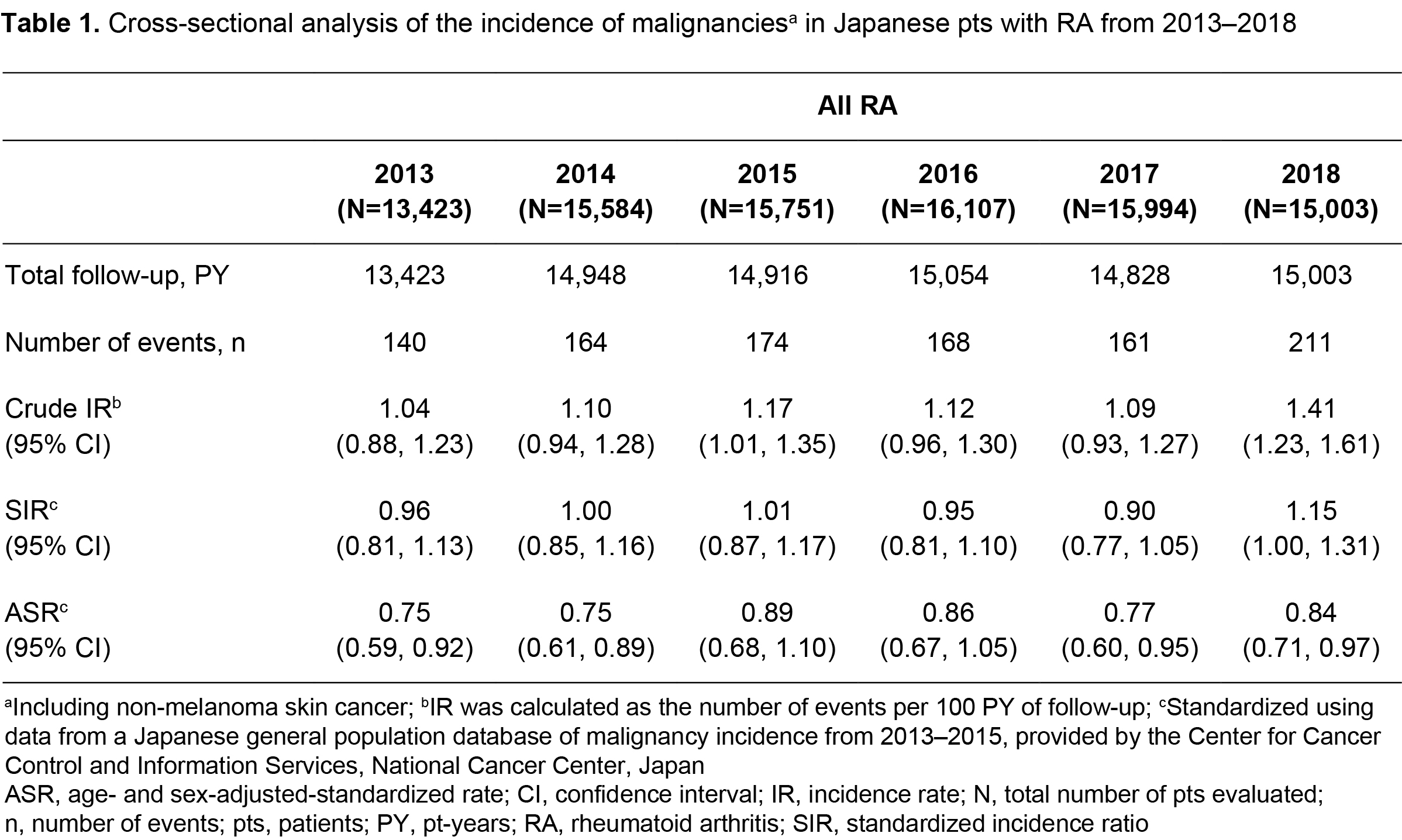
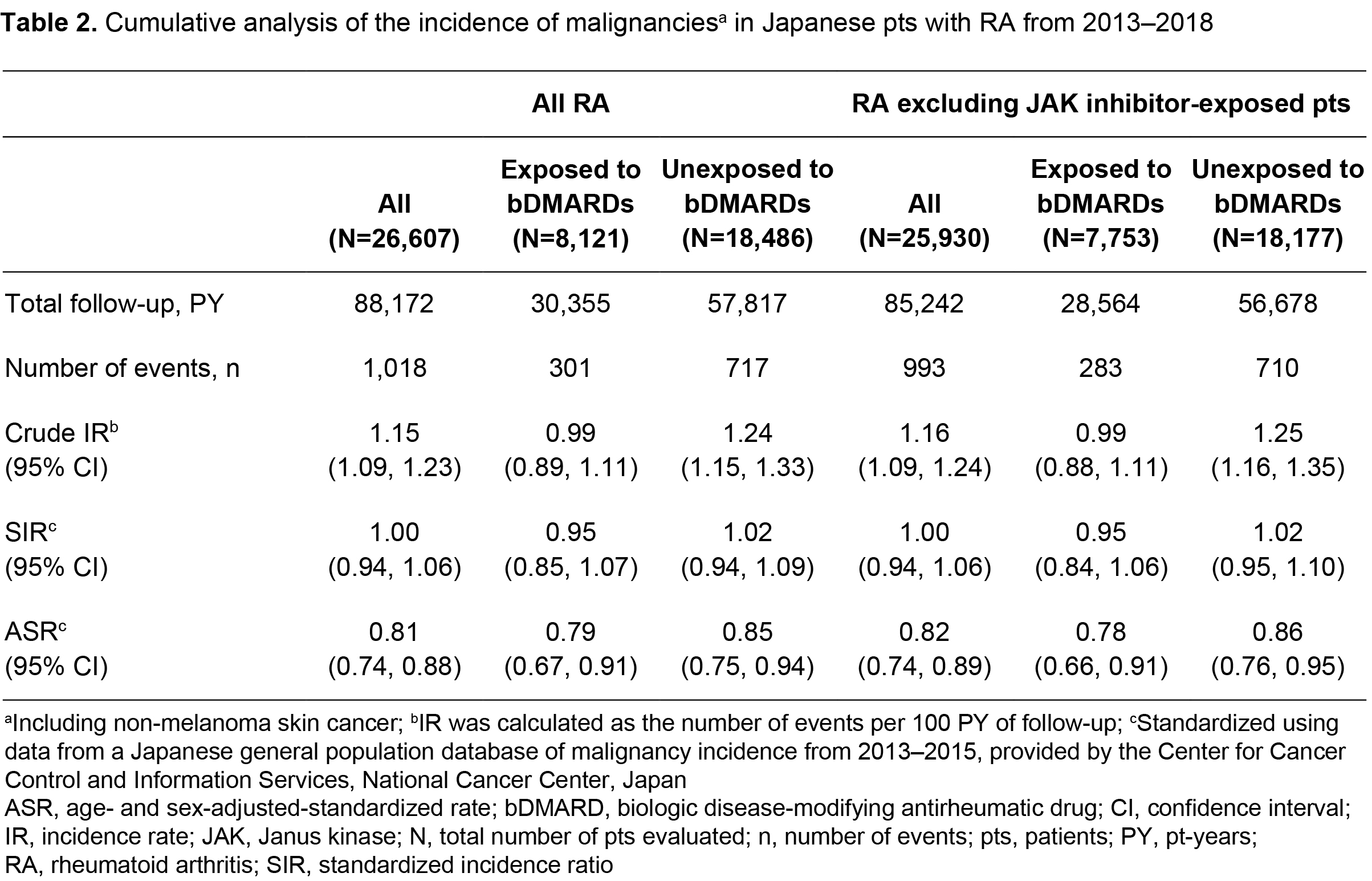
Methods: This retrospective observational study analyzed NinJa registry data for Japanese pts with RA aged ≥ 18 years with ≥ 1 data entry between 2013 (first JAK inhibitor approval for RA in Japan) and 2018. The overall cohort included all pts with RA, and two sub-cohorts were analyzed: pts exposed and unexposed to bDMARDs (exposure defined as ≥ 1 bDMARD reported in the database). Crude incidence rates (IRs) for malignancies (including non-melanoma skin cancer) were calculated as the number of events per 100 pt-years of follow-up (time between start of follow-up and end of the observation period, or withdrawal from the database). The most recent data for incidence of malignancy in the Japanese general population (2013–2015 data from the National Cancer Center, Japan) were used to calculate standardized incidence ratios (SIRs) and age‑ and sex-adjusted standardized rates (ASRs) for malignancies. Cross‑sectional (per calendar year) and cumulative analyses were performed for the overall cohort. Cumulative rates were also calculated for the sub‑cohorts, and all cumulative analyses were repeated excluding pts exposed to JAK inhibitors (ie ≥ 1 JAK inhibitor reported in the database).
Results: Data were collected for 26,607 Japanese pts with RA from 2013–2018. In the cross-sectional analysis (Table 1), the SIR and ASR for malignancies in all pts with RA were generally consistent from 2013–2018. In the cumulative analysis (Table 2), the SIR (95% confidence interval [CI]) for malignancies from 2013–2018 was 1.00 (0.94, 1.06) in all pts with RA, and 0.95 (0.85, 1.07) and 1.02 (0.94, 1.09) in pts exposed and unexposed to bDMARDs, respectively. Adjusting for age and sex, the cumulative ASR (95% CI) for malignancies from 2013–2018 was 0.81 (0.74, 0.88) in all pts with RA, and 0.79 (0.67, 0.91) and 0.85 (0.75, 0.94) in pts exposed and unexposed to bDMARDs, respectively (Table 2). In all cohorts, the cumulative SIR and ASR were similar when pts exposed to JAK inhibitors were excluded (Table 2).
Conclusion: The incidence of malignancies in Japanese pts with RA, registered in the NinJa database from 2013–2018, was similar to that in the Japanese general population. The SIR and ASR for malignancies were comparable in pts exposed and unexposed to bDMARDs. In all cohorts, rates did not increase when pts exposed to JAK inhibitors were included.
- Dougados M et al. Ann Rheum Dis 2014; 73: 62-68.
- Parikh-Patel A et al. Cancer Causes Control 2009; 20: 1001-1010.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Gemma Turner, CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Matsui T, Sugiyama N, Kawaguchi T, Kamezaki A, Toyoizumi S, Matsuyama F, Murata T, Urata Y, Kawahata K, Tohma S. Incidence of Malignancies in Japanese Patients with Rheumatoid Arthritis: Data from a Large Japanese National Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/incidence-of-malignancies-in-japanese-patients-with-rheumatoid-arthritis-data-from-a-large-japanese-national-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-malignancies-in-japanese-patients-with-rheumatoid-arthritis-data-from-a-large-japanese-national-registry/