Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: New Zealand black and white F1 (NZBW/F1) is a classic mouse model of systemic lupus erythematosus (SLE). Type I interferon (IFN) infusion accelerates lupus proteinuria and nephritis. miR-146a is a potent immune regulator to suppress inflammatory responses in T and dendritic cells. Potential involvement of this miRNA in lupus pathogenesis has been implicated in human SLE by both function and genetics. This study explored the novel functions and therapeutic potential of miR-146a in a mouse model of accelerated SLE.
Methods: At 15 weeks, NZBW/F1 mice were infused with IFNα delivered by subcutaneously implanted osmotic pumps. The miR-146a (n = 8) or PBS (n = 7) administration began one week before the IFNα infusion and lasted for 9 weeks. Age matched NZBW/F1 mice were used as negative controls (n=10). Urine samples were tested for proteinuria by dipstick. Blood sera were used for autoantibody profiling. Gene expression profile data were collected from peripheral blood RNAs of both miR-146a and PBS treated groups. At the end of 9-week intervention, the kidney tissues were harvested and renal damage was histologically investigated by HE and IgG1 staining.
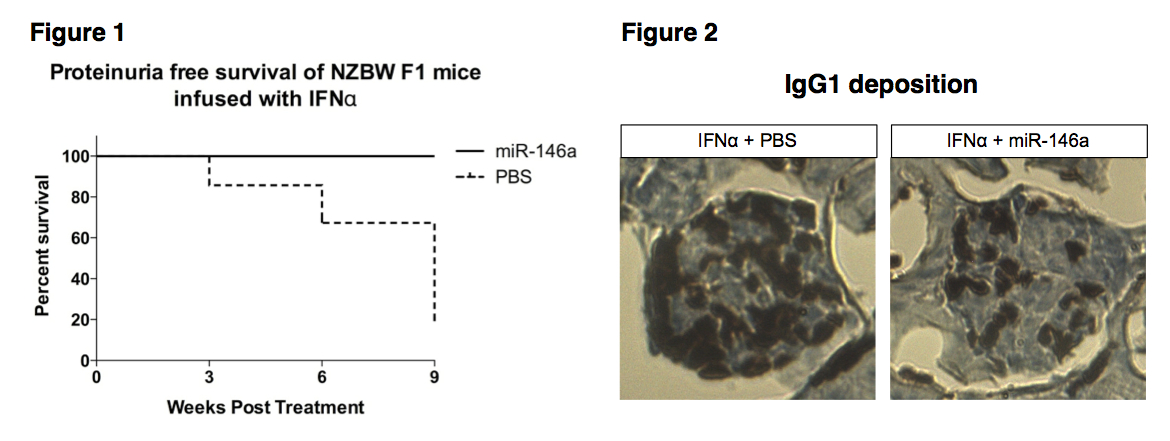
Results: Administration of IFNα in NZBW/F1 mice resulted in an accelerated lupus. Proteinuria free survival rate differs between miR-146a treated and PBS-treated groups, with a significant delayed onset of proteinuria observed in the miR-146a-treated group (p < 0.01, Fig.1). IHC staining showed reduced IgG1 deposition in kidney tissues from mice treated with miR-146a, 9 weeks after miRNA treatment (Fig. 2). Gene expression profiling by microarray showed that miR-146a treatment decreased expression of immunoglobulin proteins (IgG1, IgG2b, IgM, and IgA). Consistent with these findings, autoantibody profiling of mouse sera showed that IFNα infusion triggered an induction of autoantibodies reactive with different glomerular or glomerular basement membrane antigens. However, the expression of some lupus relevant autoantibodies (anti-H3, anti-H4, anti-histone (total), anti-LC1, anti-MPO, anti-Ribophosphoprotein P1, anti-Sm-D1 and anti-U1-snRNP-BB') was significantly suppressed following miR-146a treatment.
Conclusion: Overall, treatment with miR-146a attenuated lupus nephritis in the IFNα infused NZBW/F1 mice. The results are a proof of principal suggesting that interference with miR-146a may be a promising therapeutic strategy for treatment of lupus patients with renal involvement.
Disclosure:
D. Liang,
None;
S. Zhou,
None;
Z. Liu,
None;
Z. Shan,
None;
P. Brohawn,
None;
Y. Yao,
None;
I. Raman,
None;
Q. Z. Li,
None;
J. B. Harley,
None;
N. Shen,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-vivo-mir-146a-administration-ameliorates-murine-lupus-nephritis/