Session Information
Date: Sunday, November 8, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Miscellaneous Rheumatic Diseases
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported outcome measures (PROMs) are powerful tools which can highlight the patient experience of illness. Although PROMs are standard metrics in SLE clinical research, they are not routinely integrated in clinical care. Individuals with SLE are enthusiastic about the potential of PROMs to facilitate patient-centered care, but little is known about the utility and burden of implementing PROMs in SLE clinical encounters. The aim of this study was to assess the feasibility and impact of implementing web-based PROMs in the routine clinical care of outpatients with SLE.
Methods: Outpatients fulfilling 1997 ACR SLE classification criteria were enrolled in this longitudinal cohort study at two academic medical centers. Subjects completed PROMIS computerized adaptive tests at enrollment and prior to two consecutive routinely scheduled rheumatology visits using the ArthritisPower research registry mobile or web-based application. Score reports were shared with patients and providers before the visits. Patients and rheumatologists completed post-visit surveys evaluating the utility of PROMs in the clinical encounters.
Results: A total of 105 SLE patients and 16 rheumatologists participated in the study (Table 1). Subjects completed PROMs in 159 of 184 eligible encounters (86%, 95% CI 81 – 91) prior to study suspension due to the COVID-19 pandemic. Following baseline surveys, PROMs were completed for 90% (95% CI 82 – 95) of visit 1’s and 82% (95% CI 72 – 90) of visit 2’s. Nearly all PROMs (93%) were completed remotely. Patients and rheumatologists found that PROMs were useful (91% and 83% of encounters respectively) and improved communication (86% and 72%) (Table 2). Rheumatologists reported that PROMs impacted patient management in 51% of visits, primarily by guiding conversations (84%), but also by influencing medication changes (15%) and prompting referrals (10%). There was no statistically significant difference in visit length before (mean=19.5 minutes) and after (mean=20.4 minutes) implementation of PROMs (p=0.52).
Conclusion: The remote capture and subsequent integration of PROMs into clinical care was feasible in this diverse cohort of SLE outpatients. PROMs were useful to both patients and rheumatologists, promoting patient-centered care primarily by facilitating communication. The implementation of PROMs was not significantly associated with an increase in length of visits.
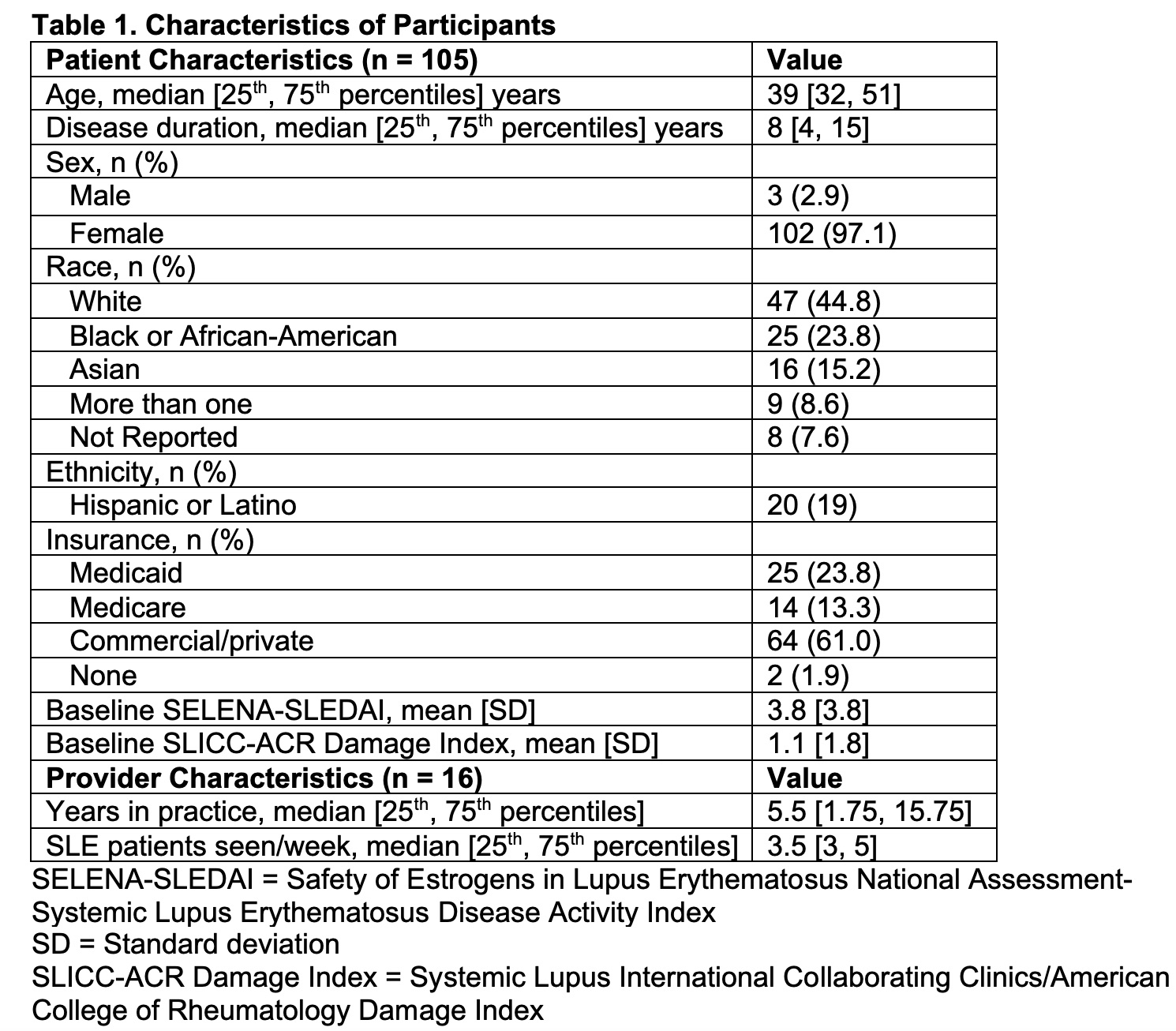 Table 1. Characteristics of Participants
Table 1. Characteristics of Participants
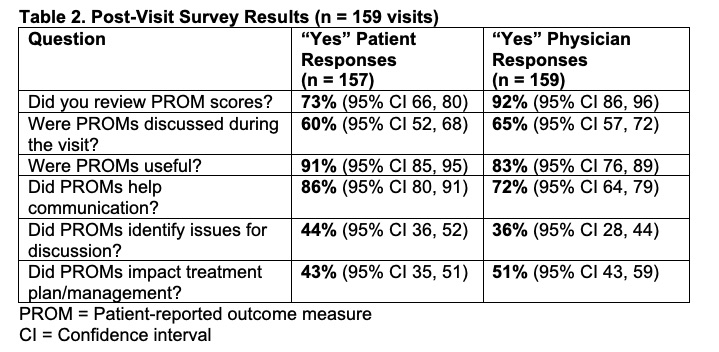 Table 2. Benefits, Challenges, and Ideal Use of Patient-Reported Outcome Measures in Clinical Care
Table 2. Benefits, Challenges, and Ideal Use of Patient-Reported Outcome Measures in Clinical Care
To cite this abstract in AMA style:
Kasturi S, Price L, Curtis D, Nowell W, Terrin N, Salmon J, Mandl L, McAlindon T. Implementation of Web-Based Patient-Reported Outcome Measures (PROMs) in SLE Clinical Care: A Multi-Center Prospective Cohort Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/implementation-of-web-based-patient-reported-outcome-measures-proms-in-sle-clinical-care-a-multi-center-prospective-cohort-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implementation-of-web-based-patient-reported-outcome-measures-proms-in-sle-clinical-care-a-multi-center-prospective-cohort-study/
