Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Sprifermin, recombinant human fibroblast growth factor 18 (rhFGF-18), is being investigated as a potential disease-modifying osteoarthritis drug. The Phase 2 FORWARD study demonstrated that intra-articular (IA) sprifermin significantly and dose-dependently improved cartilage thickness in knee OA (KOA).Cartilage defects, the most severe of which present as dABs, are known to increase in size with KOA progression and be associated with KOA pain and incident knee replacement. This analysis investigates the impact of sprifermin treatment on dABs in KOA.
Methods: In FORWARD, participants (pts) were randomized 1:1:1:1:1 to 3 weekly IA injections (1 cycle) of either PBO or sprifermin 30 µg or 100 µg every 6 or 12 months (Q6M, Q12M) for 18 months, then followed through year 5. The modified (with baseline [BL] and ≥1 post-BL MRI) intent-to-treat (mITT) and subgroup at risk (mSAR, enriched for more severe KOA [BL WOMAC Pain ≥40, minimum medial or lateral joint space width {mJSW} 1.5-3.5 mm]) populations were analyzed. dABs, derived from manual, quality-controlled cartilage and subchondral bone segmentations, were computed as the percentage (%) of the total area of subchondral bone not covered by cartilage of femorotibial joint (FTJ) regions. Measurements were analyzed at BL and Week 104 (W104) for the a) 30 and 100 μg arms individually, b) 30 and 100 μg arms combined, and c) pts with BL dAB. No imputation was performed for missing data; only descriptive data are provided.
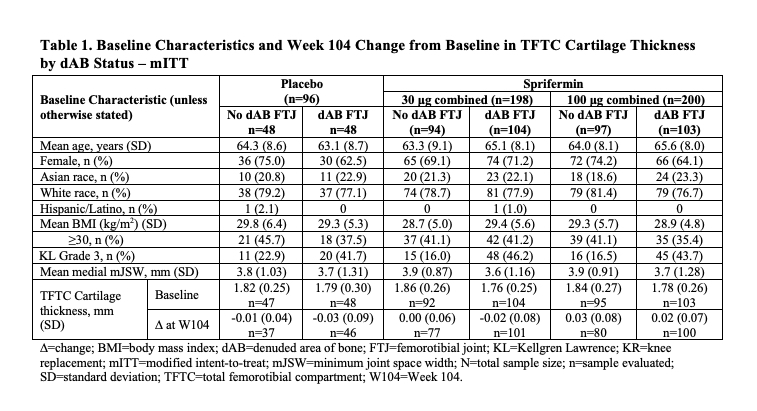
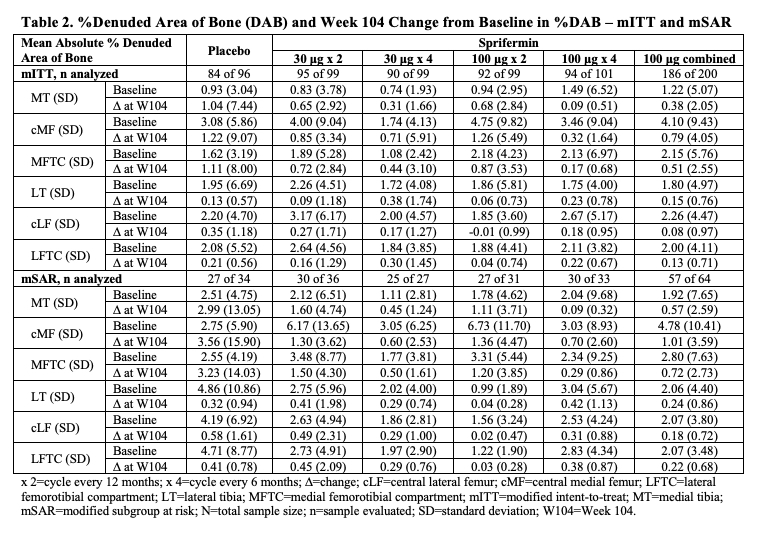
Results: Characteristics at BL showed 52% (n=255/494) of the mITT population had some FTJ dAB (Table 1); in the SAR, 61% (n=98/161). Those with FTJ dAB at BL had more severe radiographic disease by Kellgren-Lawrence (KL) grade (44.3% vs 17.6% KL3) and medial mJSW (3.6 mm vs 3.9 mm).Baseline dABs were associated with more structural progression by quantitative cartilage thickness measures, independent of treatment arm (Table 1). Table 2 presents the %dAB and absolute change from BL (CFB) in %dAB data for the 6 FTJ regions analyzed. Of these, the weight-bearing (central) medial femur (cMF) showed the greatest %dAB (range 1.74-4.75) in the different treatment arms at BL in the mITT (Table 2). In the medial compartment, the absolute %dAB CFB at W104 also tended to be greater (worse) in the PBO- (range 1.04-1.22) vs the sprifermin-treated arms (eg, range 0.38‑0.79 for sprifermin 100 μg combined), with the cMF showing the greatest differentiation. The degree of the differences in longitudinal changes between treatment arms in the medial compartment was more pronounced in the mSAR than mITT population, eg, W104 CFB of cMF %dAB mSAR 3.56 vs 1.01 and mITT 1.22 vs 0.79 in the PBO vs sprifermin 100 μg combined group. Yet, little difference was seen in the longitudinal changes in %dAB between the sprifermin- and PBO-treated arms in the lateral compartment.
Conclusion: The presence of dAB appears to be associated with more severe radiographic OA and structural progression of KOA. Sprifermin treatment, particularly at the highest dose, appears to slow the increase (worsening) of dAB compared to PBO, especially in the medial compartment. Further evaluation of these findings and the relationship of dAB with symptomatic outcomes is warranted, including in prospective clinical trials.
To cite this abstract in AMA style:
Eckstein F, Wirth W, Conaghan P, Hochberg M, Guermazi A, Andrade J, Zhao L, Goel N. Impact of Sprifermin on Denuded Areas of Subchondral Bone (dABs): A Post Hoc Analysis of the FORWARD Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-sprifermin-on-denuded-areas-of-subchondral-bone-dabs-a-post-hoc-analysis-of-the-forward-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-sprifermin-on-denuded-areas-of-subchondral-bone-dabs-a-post-hoc-analysis-of-the-forward-study/