Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: When deciding to start patients with PsA on biologic therapy, rheumatologists may focus on skin involvement in addition to joint symptoms. The purpose of this study is to investigate the clinical significance of skin involvement in patients with PsA newly initiating an advanced therapy.
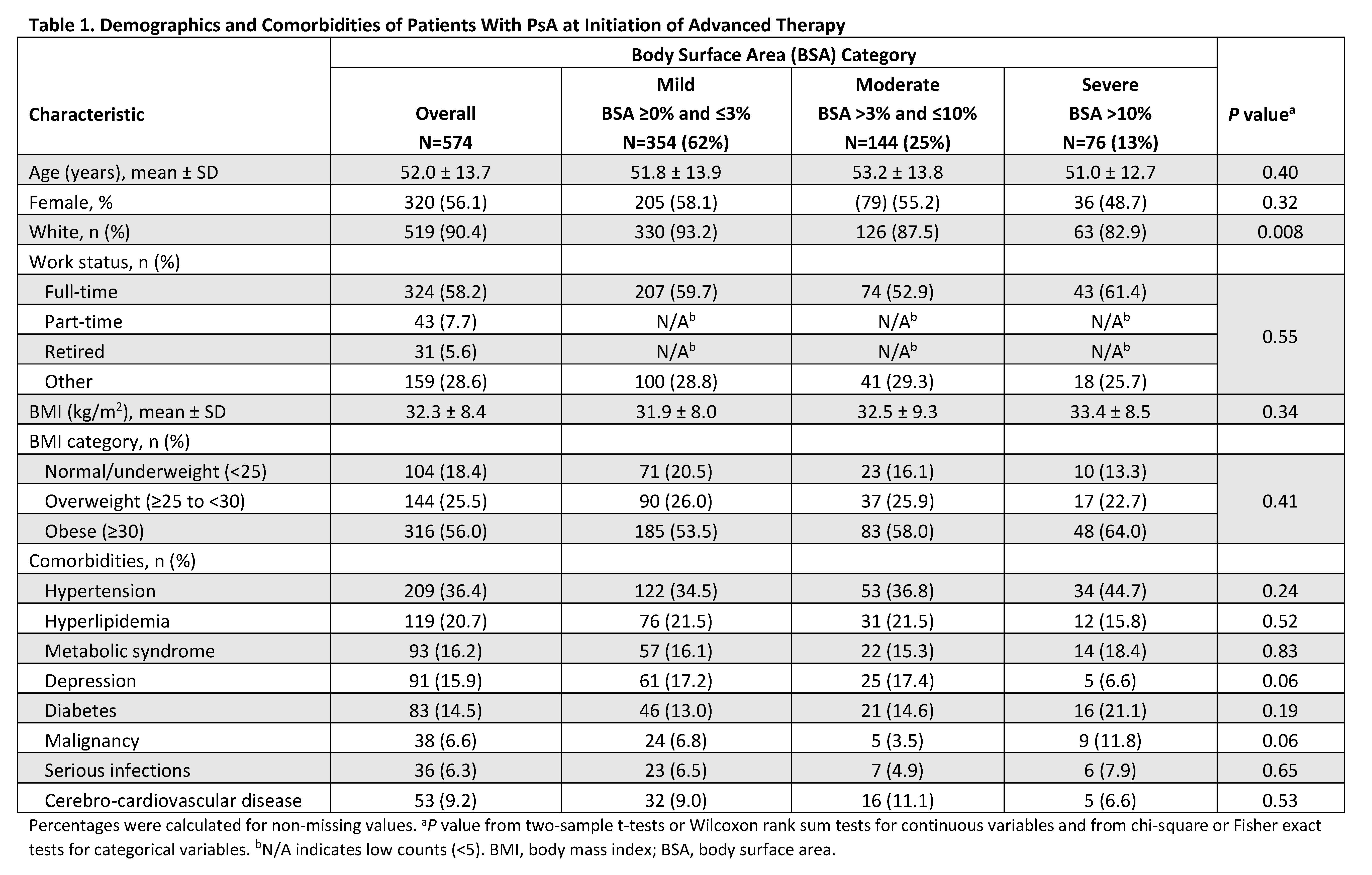
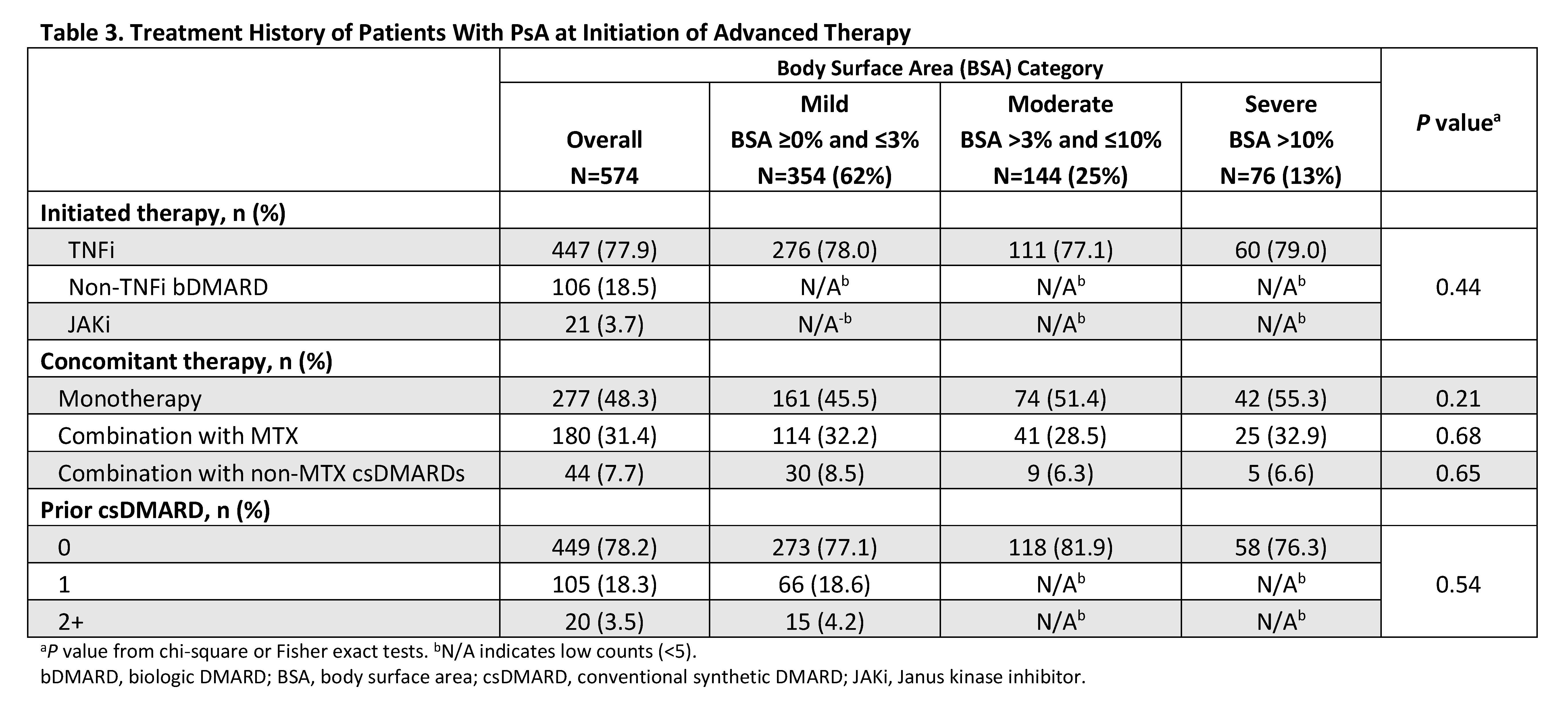
Methods: This was a descriptive analysis of adult patients (≥18 years) with PsA enrolled in the Corrona PsA/SpA Registry between March 2013 and March 2020. Patients were included if they initiated an advanced therapy (i.e., biologic or targeted synthetic disease-modifying antirheumatic drug) at enrollment, had a body surface area (BSA) measurement, and had no prior history of advanced therapy at initiation. Baseline demographics, clinical characteristics, treatment history, disease activity measures, and patient-reported outcome measures were stratified by BSA categories of mild (BSA ≥0 and ≤3%), moderate (BSA >3% and ≤10%), and severe (BSA >10%). Continuous measures were reported using means and standard deviation; means for BSA groups were compared using two-sample t-tests or Wilcoxon rank sum tests. Categorical measures were reported as frequencies and percentages; frequencies in BSA groups were compared using chi-square or Fisher exact tests.
Results: A total of 574 eligible initiators included 354 (61.7%), 144 (25.1%), and 76 (13.2%) with mild, moderate, and severe skin involvement, respectively. Across BSA groups, mean age was 51.0–53.2 years and 48.7–58.1% were female (Table 1). TNF inhibitors were the most commonly initiated biologic (77.1–79.0%). Overall, BSA groups were very similar across baseline demographics and comorbidities. Across BSA groups, mean baseline scores were generally similar (P>0.05) for musculoskeletal PsA domains (tender/swollen joint, dactylitis, enthesitis counts, and axial involvement), disease activity measures (Clinical Disease Activity Index, Disease Activity in Psoriatic Arthritis, and Psoriatic Arthritis Disease Activity Score), and patient-reported outcomes of pain, Health Assessment Questionnaire-Disability Index, the Assessment of SpondyloArthritis international Society Health Index, and impairment of work productivity and ability to perform daily activities (Table 2). BSA groups also did not differ significantly on prior or current therapy at baseline (Table 3).
Conclusion: The majority of patients with PsA who initiated an advanced therapy had mild to moderate skin involvement. Moreover, baseline demographics, disease activity in musculoskeletal PsA domains, and patient-reported outcomes were comparable across mild to severe skin involvement. Although rheumatologists’ treatment decisions can be driven by many factors, severe psoriasis is relatively uncommon in rheumatology practice. Thus, musculoskeletal symptoms are more likely to drive therapeutic decision making when newly initiating advanced therapies for patients with PsA.
Medical writing services provided by Joann Hettasch of JK Associates, Inc. (a Fishawack Health Company) and funded by AbbVie.
To cite this abstract in AMA style:
Mease P, McLean R, Blachley T, Anatale-Tardiff L, Saffore C, Lovan C, Ogdie A. Impact of Skin Involvement on Disease Burden Among Patients with Psoriatic Arthritis: Data from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-skin-involvement-on-disease-burden-among-patients-with-psoriatic-arthritis-data-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-skin-involvement-on-disease-burden-among-patients-with-psoriatic-arthritis-data-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/