Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Current rheumatology treatment guidelines recommend lab monitoring for methotrexate toxicity at three-month intervals for established patients. We sought to evaluate whether monitoring labs at six-month intervals instead of recommended intervals was associated with more frequent reporting of lab test abnormalities.
Methods: We performed a retrospective analysis on patients at a large academic medical center who were taking methotrexate and who had at least two laboratory measurements of ALT, AST, PLT, WBC, MCV or HCT. Data for 3,821 patients spanning 2017–2024 were extracted from the electronic health record. Query parameters included patient MRN, sex, BMI, birth date, encounter date, methotrexate prescriptions, HCT, MCV, PLT, WBC, ALT, and AST. Only encounters in which the patient was prescribed MTX more than 90 days prior were analyzed in order to exclude patients new to methotrexate. We plotted the changes in lab values from one encounter to the next against their respective test intervals and conducted linear regression analysis. We defined two different testing intervals: 3 months (2-4 months) and 6 months (5-7 months) and tested whether there was a difference in the mean change in lab value between the two testing intervals. We tested whether patients were more likely to transition from a clinically normal lab value to a lab value exceeding a clinically relevant threshold in either testing interval group.
Results:
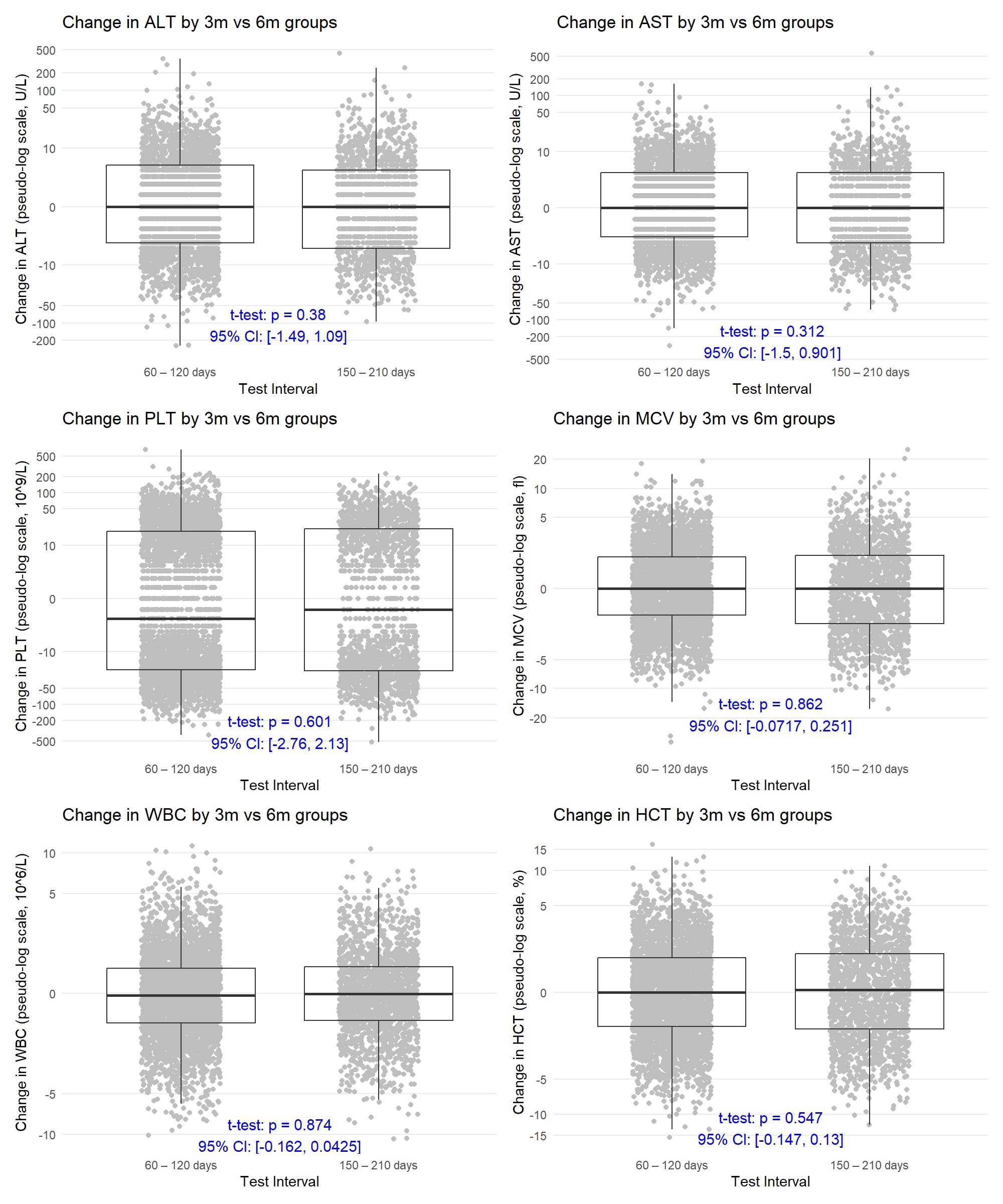
Results: There was no correlation between test frequency and ALT, AST, WBC, HCT, MCV or PLT count (R-squared < 0.01, Figure 1). Additionally, the average change between tests in these lab values was not different when measured every six months compared to every three months (Table 2). Patients tested at six months were not more likely to develop an abnormal lab result after a previously normal result compared to patients tested at three months (Figure 2).
Conclusion: Monitoring routine labs for methotrexate therapy using a six-month interval was not worse than monitoring at three months for detecting impactful lab abnormalities. The current ACR guideline to perform the measurements at three months may not be necessary and should be re-evaluated for cost-effectiveness and unnecessary patient burden.
 Linear regression of test level changes vs. time interval between tests in patients taking MTX longer than 90 days
Linear regression of test level changes vs. time interval between tests in patients taking MTX longer than 90 days
.jpg) Comparing changes in LFT and CBC across 2 different testing intervals: 60 – 120 days and 150 – 210 days for patients taking MTX longer than 90 days. A one-tailed t-test was performed with the p-value reported on the figure and a two-tailed 95% confidence interval for the difference in means was calculated.
Comparing changes in LFT and CBC across 2 different testing intervals: 60 – 120 days and 150 – 210 days for patients taking MTX longer than 90 days. A one-tailed t-test was performed with the p-value reported on the figure and a two-tailed 95% confidence interval for the difference in means was calculated.
.jpg) Table 1. Percent of lab values starting in a clinically normal range that crossed a clinically concerning threshold in the subsequent test for two different testing intervals representing 3 months and 6 months in patients taking MTX longer than 90 days. Odds-ratio that the longer interval was associated with abnormal lab values adjusted by age, sex and BMI is also reported. The upper limit of normal (ULN) was established as 35 U/L (females) or 50 U/L (males) for ALT, as 50 U/L for AST. The lower limit of normal (LLN) was established as than 35% (female) or 37% (male) for HCT.
Table 1. Percent of lab values starting in a clinically normal range that crossed a clinically concerning threshold in the subsequent test for two different testing intervals representing 3 months and 6 months in patients taking MTX longer than 90 days. Odds-ratio that the longer interval was associated with abnormal lab values adjusted by age, sex and BMI is also reported. The upper limit of normal (ULN) was established as 35 U/L (females) or 50 U/L (males) for ALT, as 50 U/L for AST. The lower limit of normal (LLN) was established as than 35% (female) or 37% (male) for HCT.
To cite this abstract in AMA style:
Simko S, Mukherjee S, Zhang R, Bajaj P, Bermas B. Impact of Six-Month Monitoring Compared to Three-Month Monitoring of Labs for Methotrexate Toxicity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-six-month-monitoring-compared-to-three-month-monitoring-of-labs-for-methotrexate-toxicity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-six-month-monitoring-compared-to-three-month-monitoring-of-labs-for-methotrexate-toxicity/
