Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is widely used for rheumatic diseases, but data on its cardiac safety, particularly QTc prolongation, remains limited. Concerns about HCQ inducing QTc prolongation, which can lead to Torsades de pointes and sudden death, arose during the COVID-19 pandemic, though extrapolating from sick patients with COVID to patients with stable RMD patients remains unclear.
Methods: We abstracted data from Veterans with RA, SLE, or Sjögren’s syndrome (based on ICD-10 diagnostic codes) from 3 VA sites (Sept 2016–Mar 2022), aged 18–85 with ≥1 ECG. Bazett-corrected QTc were abstracted. ECGs with Afib/flutter, pacing, or 300 > QTc > 600 ms were excluded. To define exposure to HCQ, windows were defined using days supplied on dispensed prescriptions (and then multiplied by 1.2 so as not to assume 100% adherence). Primary outcome was QTc > 500 ms. Secondary QTc outcomes included QTc > 400 ms and QTc mean. Multivariate models adjusted for age, gender, race/ethnicity, comorbidities, and concomitant QT-prolonging drugs. For a subset of Veterans from the LA VA, we had HCQ blood levels and correlated with QTc (ECG obtained within 60-days of the lab value).
Results: HCQ use was highest in Veterans with SLE (63%), followed by RA (41%) and Sjögren’s (14%). Across the 3 VA sites, 4,774 Veterans had 24,549 ECGs. Of 3,157 Veterans never on HCQ, 5.4% of ECGs showed QTc >500 ms. 769 veterans were on HCQ at some time during the study period, but never on HCQ when ECG was obtained, and had the lowest proportion of QTc > 500 ms (3.7%). Veterans who were intermittently off and on HCQ at the time of ECG recordings had the highest proportion of QTc >500 (7.2% and 8.5%, respectively). While Veterans who were always on HCQ at time of ECG had similar proportion (5.7%) of QTc >500 to those Veterans never on HCQ. Differences between the proportion of QTc prolongation < 500 ms did not differ statistically across the HCQ exposure groups. Differences between the groups were statistically significant for the secondary outcomes of QTc > 400 ms and QTc mean (Refer to Table 2). Among 35 patients with paired HCQ blood levels and ECGs, for each 100 ng/mL rise in HCQ level was linked to a 9.4 ms increase in QTc (r = 0.50, p = 0.0005). Eg. 70 kg patient taking 200 mg daily (2.9 mg/kg/day) might see a 6–12 ms QTc increase. This suggests a modest, dose-dependent QTc effect, consistent across cohorts. Of note, none of the 35 patients had a therapeutic HCQ level (suggestive of low adherence). Poor adherence was further noted in our overall cohort with a low median annual MPR of 72% yielding a median estimated dose of 2.3 mg/kg/day.
Conclusion: In this large, multi-site VA study involving patients with rheumatic diseases, HCQ use was associated with a small but statistically significant, dose-dependent QTc prolongation. Although the absolute QTc increase was small and unlikely to affect most patients, these results support targeted ECG monitoring, particularly in older or female patients, or in those receiving other QT prolonging medications or with CKD or cardiac comorbidities. These results also suggest the potential value of HCQ drug level monitoring where the detection of supratherapeutic HCQ levels should prompt ECG evaluation.
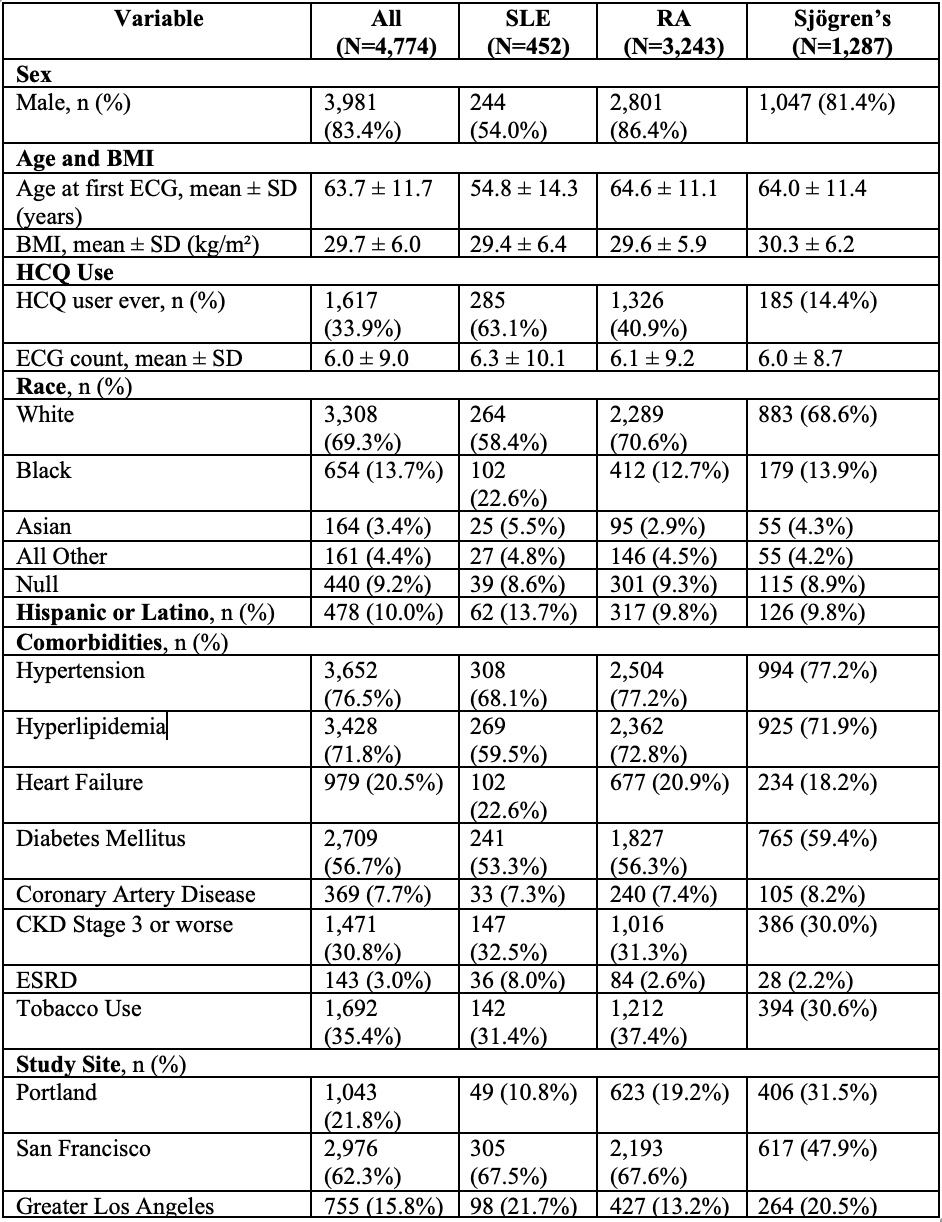 Baseline Characteristics (MUSE Cohort)
Baseline Characteristics (MUSE Cohort)
.jpg) Adjusted QTc outcomes (MUSE Cohort)
Adjusted QTc outcomes (MUSE Cohort)
To cite this abstract in AMA style:
Ibrahim M, Good S, Tran V, Chetrit D, McClean M, Sim M, Kang H, Barton J, Fang M, Gaffo A, Hage f, Jackevicius c, Pillinger M, Schmajuk g, Singh J, Warner A, Yaun N, FitzGerald J. Impact of Hydroxychloroquine Usage on QTc in Veterans with Rheumatic Musculoskeletal Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-hydroxychloroquine-usage-on-qtc-in-veterans-with-rheumatic-musculoskeletal-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-hydroxychloroquine-usage-on-qtc-in-veterans-with-rheumatic-musculoskeletal-disease/
