Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In clinical trials, biologic disease-modifying antirheumatic drugs (bDMARDs) for juvenile idiopathic arthritis (JIA) have demonstrated good efficacy. However, less is known about the impact of the increasing use of bDMARDs on JIA outcomes outside of clinical trial settings. We examined selected outcomes among children with JIA after bDMARD availability compared to those prior to bDMARD availability.
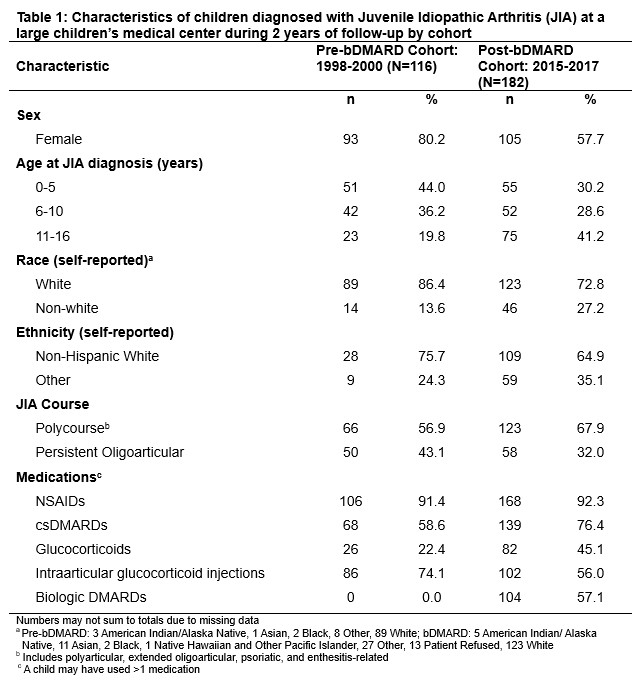
Methods: This retrospective cohort study assessed two JIA cohorts representing pre- and post-bDMARD availability for achievement of, elapsed time until, and time in inactive disease, along with disease flare after achieving inactive disease status. The pre-bDMARD cohort included children diagnosed with JIA 1998-2000 (N=116); the post-bDMARD cohort included children diagnosed 2015-2017 (N=182). Diagnosis dates and outcomes were abstracted for children with 2 years of follow up post-diagnosis. Mean days to inactive disease and time in inactive disease were compared using t-tests. Poisson regression estimated relative risks (RRs) and 95% confidence intervals (CIs) for outcomes post- compared to pre-bDMARD availability, adjusting for diagnosis age, JIA category (polycourse vs persistent oligoarthritis), and receipt of intraarticular glucocorticoid injection.
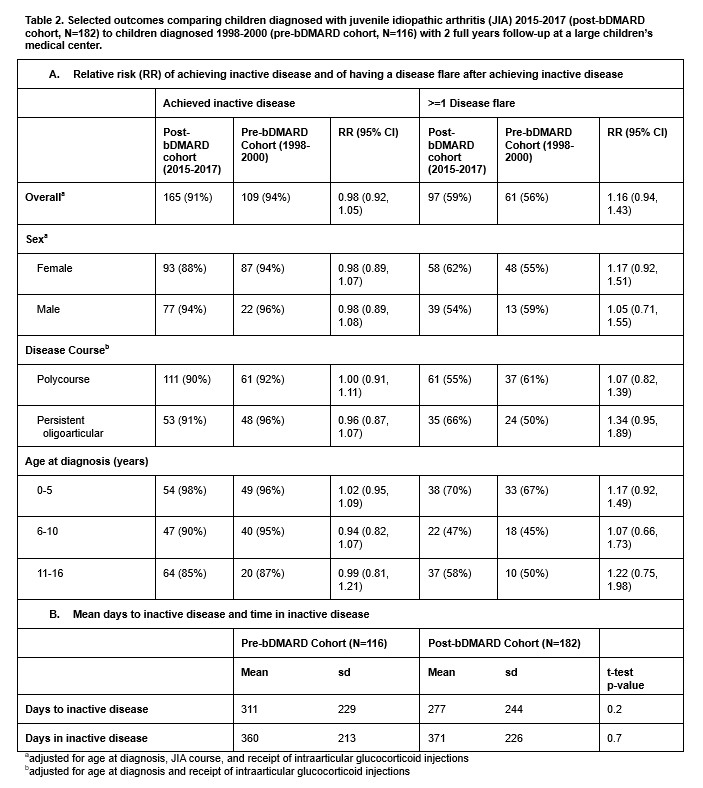
Results: Children in the post-bDMARD cohort were more likely to be older, male, with polycourse arthritis, and more likely prescribed conventional DMARDs and glucocorticoids but less likely to have intraarticular glucocorticoid injections. 57% of children in the post-bDMARD cohort were prescribed a bDMARD during follow up. Mean elapsed time to inactive disease in the post-bDMARD cohort was 277 days (SD+244) vs 311 days (SD+299) in the pre-bDMARD cohort (p=0.2). Mean time in inactive disease during follow-up in the bDMARD cohort was 371 days (SD 226) vs 360 days (SD 213) in the pre-bDMARD cohort (p=0.7). Adjusted RRs of achieving inactive disease or occurrence of post-remission flare in the bDMARD cohort were 0.98 (95%CI: 0.92, 1.05) and 1.15 (95%CI 0.94-1.43), respectively. Results did not vary by age (0-5 yrs, 5-10 yrs, 11-16 yrs), sex or race/ethnicity, although flare risks for polycourse (RR 1.07, 95%CI 0.82-1.39) and persistent oligoarticular (RR 1.34, 95%CI 0.95-1.89) differed slightly.
Conclusion: Although mean days to inactive disease decreased, and mean days with inactive disease slightly increased for children in the bDMARD era, achievement of inactive disease did not significantly differ between the groups. Unexpectedly, the proportion of children with disease flares after remission was slightly, but not significantly larger in the post-bDMARD cohort, particularly for those with persistent oligoarticular JIA. Although limited by small numbers and proxy bDMARD exposure assessment, our results demonstrate a reduction in the number of days of disease-related suffering among children with JIA receiving care post-bDMARD availability, and suggest further development of targeted regimens for children with persistent oligoarticular JIA are needed.
To cite this abstract in AMA style:
Sutton A, Balay-Dustrude E, Mueller B, Shenoi S. Impact of Approval of Biologic DMARDs on JIA Outcomes in a Single Center [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-approval-of-biologic-dmards-on-jia-outcomes-in-a-single-center/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-approval-of-biologic-dmards-on-jia-outcomes-in-a-single-center/