Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The 2016 ACR/EULAR Myositis Response Criteria represent a composite measure that is increasingly being used as a primary end point in myositis clinical trials. The total improvement score (TIS) is calculated using differentially-weighted changes in six myositis core set measures, with thresholds for minimal, moderate and major improvement. It is unclear if achievement of these improvement levels truly reflects changes in symptoms, physical function, and physical activity in myositis patients. In this study, we examined the impact of achieving ACR-EULAR response criteria on patient-centered outcome measures.
Methods: Myositis patients were consecutively enrolled in an observational study with baseline, 3- and 6-month visits where six core set measures [extra-muscular, patient global, and physician global disease activity, manual muscle testing, HAQ-DI, and creatine kinase levels] were evaluated and TIS was calculated for each visit (https://www.niehs.nih.gov/research/resources/imacs/response_criteria/adult.html). The patient centered outcome measures included fatigue (VAS, SF-36), pain (VAS, SF-36), muscle strength (dynamometry), patient reported physical function (PROMIS PF-20, SF-36), task oriented physical function tests [sit-to-stand, timed up-and-go, and six-min walk test] and physical activity (Actigraph). Wilcoxon signed-rank test was used to assess significance of change in outcome measures over 3- and 6-months. Relative changes in outcome measures were compared between patients with improvement vs. those without improvement by using Mann-Whitney U test. Spearman correlation was used for correlations between TIS and changes in specified outcome measures.
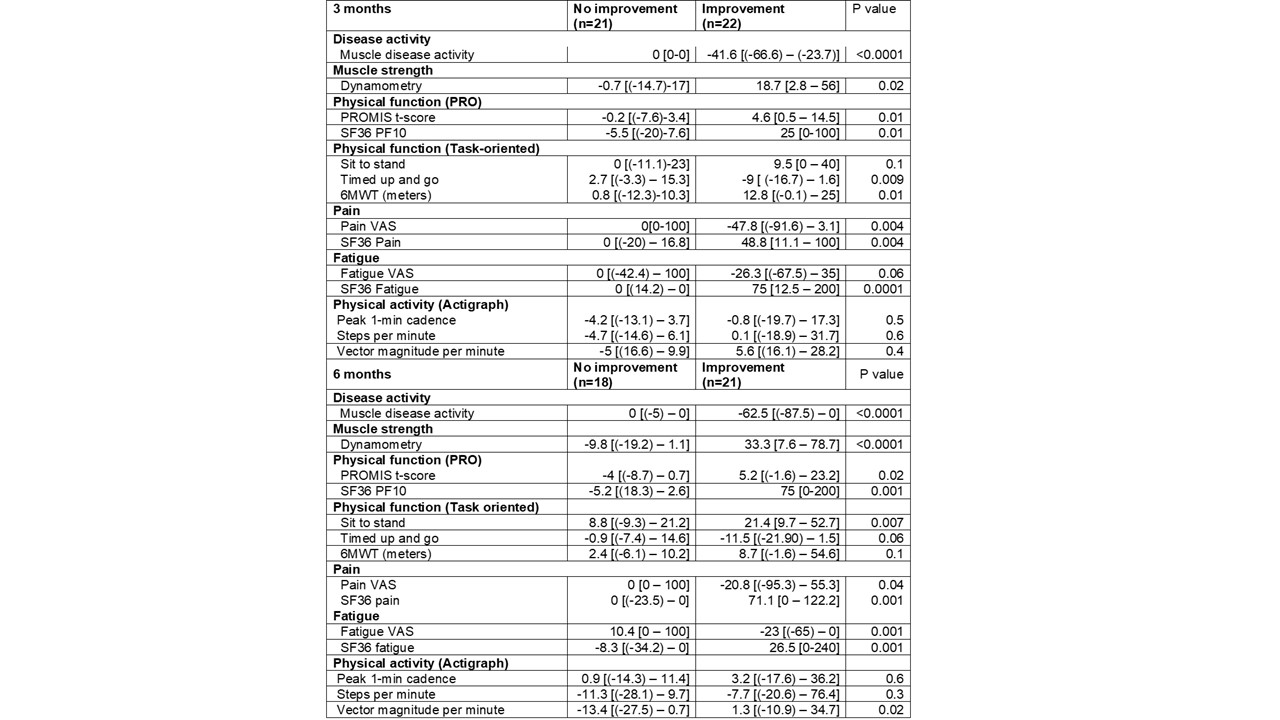
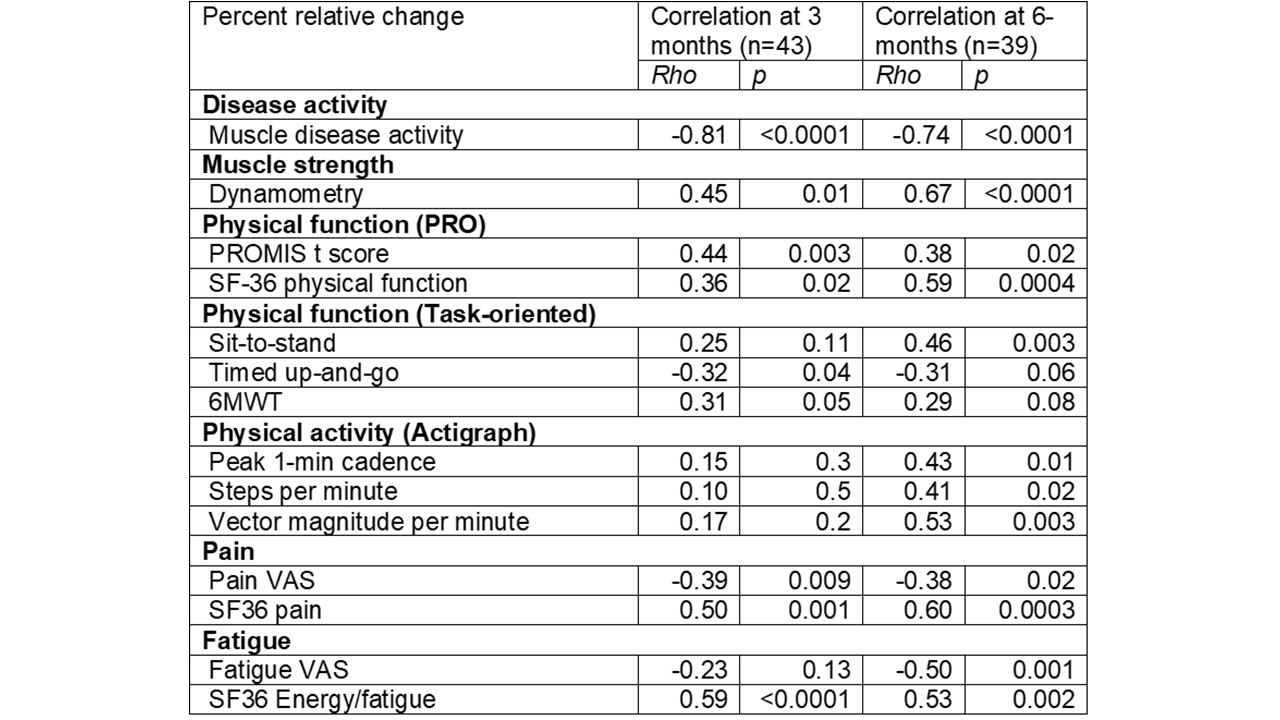
Results: Fifty myositis patients were enrolled [mean age: 52, 60% female, 94% white], including 6 PM, 24 DM, 9 necrotizing myopathy and 11 with anti-synthetase syndrome. As per ACR/EULAR criteria, 22 patients had TIS scores satisfying criteria for improvement (13 minimal, 7 moderate, 2 major) at 3-months. Patient centered outcome measures including fatigue, pain, muscle strength, patient reported, and task oriented physical function measures demonstrated significantly greater changes in patients who met ACR/EULAR response criteria of at least minimal improvement compared to those who did not (Table 1). TIS correlated moderately-strongly with relative changes in most patient-centered outcome measures (Table 2). Similar results were noted at 6-months (n=39). Percentage agreement of improvement per TIS was 62% with patient-reported improvement and 90% with physician-reported improvement at 6-months. Patients who had at least minimal improvement also demonstrated significant improvement in pain, fatigue, muscle strength, PROMIS-PF, and task-oriented physical function tests over 6-months.
Conclusion: Achieving improvement using the ACR/EULAR myositis response criteria was accompanied by significant improvement in several patient centered outcome measures including fatigue, pain, muscle strength, physical function, and physical activity. Our results reinforce the validity of the ACR/EULAR myositis response criteria and support their use as a clinically meaningful metric of improvement.
To cite this abstract in AMA style:
Saygin D, Moghadam-Kia S, Oddis C, Ascherman D, Neiman N, Koontz D, Aggarwal R. Impact of Achieving 2016 ACR/EULAR Response Criteria on Patient Centered Outcome Measures in Myositis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/impact-of-achieving-2016-acr-eular-response-criteria-on-patient-centered-outcome-measures-in-myositis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-achieving-2016-acr-eular-response-criteria-on-patient-centered-outcome-measures-in-myositis/