Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The recent discovery of highly differentiated, killer cell lectin-like receptor subfamily G member 1 (KLRG1)+ T cells in the muscle of inclusion body myositis (IBM) patients raises the potential of targeting KLRG1 for therapeutic intervention. High-resolution mapping of blood KLRG1+ T cells and the broader T cell compartment of IBM patients is lacking. Here, we report the deep immunophenotyping of IBM blood T cell compartments and the relationship of derived biomarkers to IBM diagnosis, disease duration, and disease severity.
Methods: Patients providing informed consent who met the European Neuromuscular Centre (ENMC) criteria for clinically defined or probable IBM were included. Functional data and quality of life questionnaires were collected. Serological testing for NT5C1A antibodies and analysis of markers of T cell and NK subsets including KLRG1 isolated from peripheral blood through multiplex flow cytometry were done and compared with data from age-matched healthy donors.
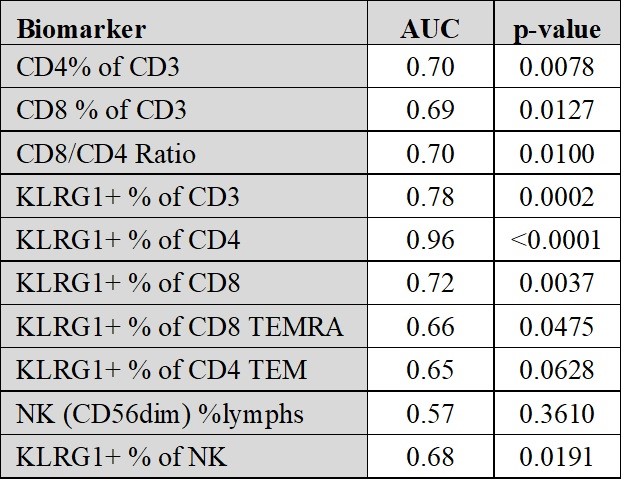
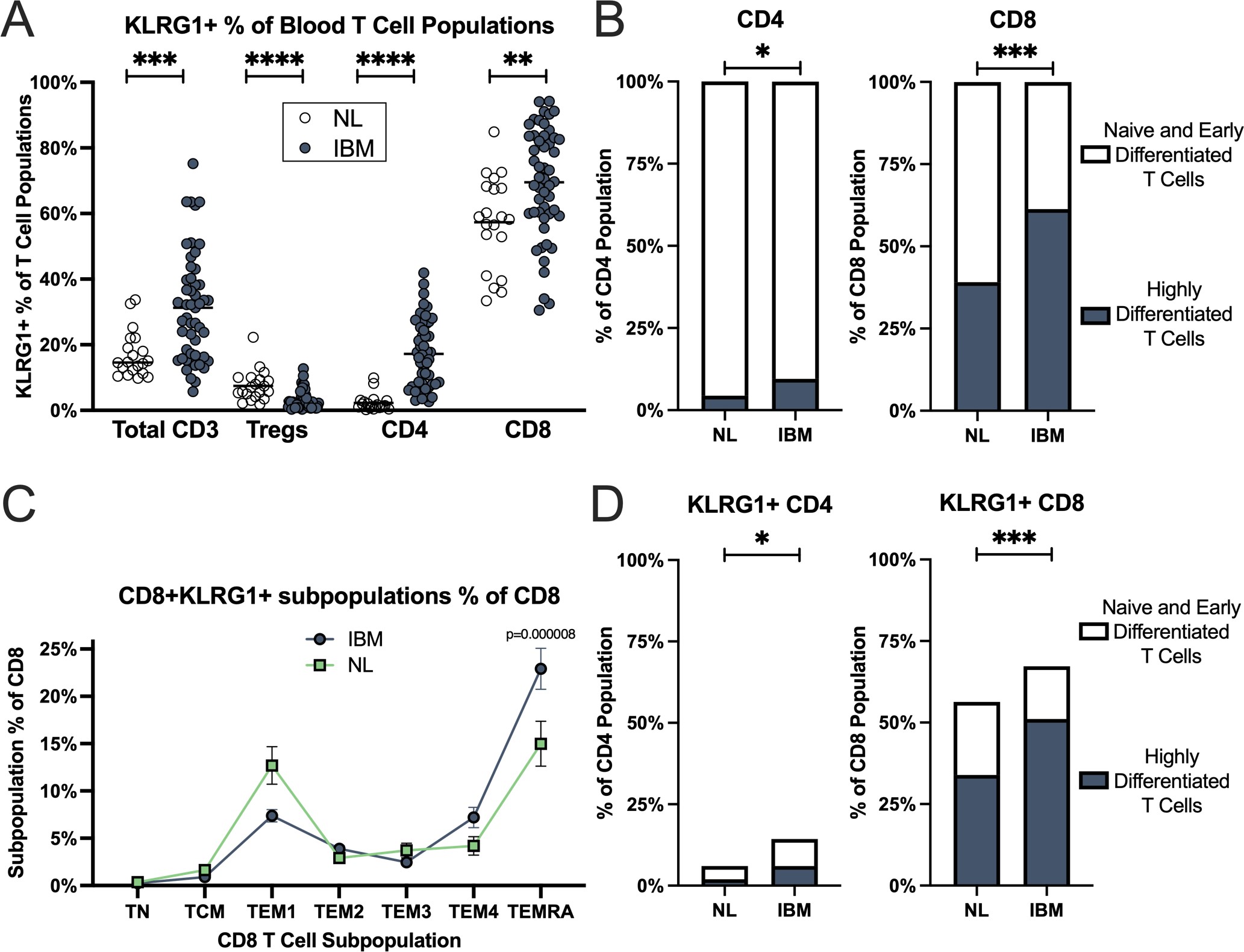
Results: Data were obtained from 51 IBM patients and 19 healthy donors. A population of KLRG1+ T effector memory (TEM) and T effector memory re-expressing CD45RA (TEMRA) cells was expanded in both the CD4+ and CD8+ T cell subpopulations of IBM patients (Figure 1). KLRG1 expression in CD8+ T cells increased with T cell differentiation with the lowest levels in naïve T cells and highest in highly differentiated TEMRA and CD56+CD8+ T cells in IBM. The CD8+ TEMRA and KLRG1+ CD8+ TEMRA proportions were not associated with IBM disease severity, but modestly positively associated with disease duration. The KLRG1+CD4+ cells in IBM patients vs healthy controls had high diagnostic performance (area under the curve=0.96; p< 0.0001) (Table 1). Minimal KLRG1 expression was present on regulatory T cells (3%) and no apparent alterations in NK cell phenotypes were identified in IBM.
Conclusion: Our findings reveal an expansion of blood KLRG1+ CD4+ and CD8+ TEMRA and TEM cells in IBM. Lack of association with disease activity suggests these cells may have a pathogenic role throughout the entire disease course. Measurement of these cells potentially allows for new blood diagnostic biomarkers.
To cite this abstract in AMA style:
Goyal N, Cauchi J, Irani T, Araujo N, Wang L, Wencel M, Li V, Greenberg S, Mozaffar T. Immunophenotyping of Inclusion Body Myositis Blood T Cells: Pathogenic and Biomarker Implications [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/immunophenotyping-of-inclusion-body-myositis-blood-t-cells-pathogenic-and-biomarker-implications/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunophenotyping-of-inclusion-body-myositis-blood-t-cells-pathogenic-and-biomarker-implications/