Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 7:30AM-9:00AM
Background/Purpose: Primary Sjögren’s syndrome (pSS) is a complex autoimmune disease with significant heterogeneity. Our study aimed to clarify the etiology and molecular variation of the target organ, salivary glands (SGs), in pSS by stratifying them into distinguishable subgroups, which could inform treatment choices in pSS.
Methods: Large-scale transcriptomic profiling of SGs was conducted on 396 pSS, 87 non-pSS, and 44 early-pSS individuals from a multicenter consecutive Chinese cohort. Unsupervised clustering methods were used for molecular classification and integrated analyses were performed to elucidate comprehensive clinical and biological features, immune-metabolic and genetic characteristics. Lymphoma risk and treatment response for pSS subgroups was also predicted for pSS subtypes.
Results: Three distinct subtypes were identified both in established and early-pSS, including “pauci-immune/C1”, “cold-immune/C2”, and “hot-immune/C3”. Pauci-immune/C1 exhibited a similar biologic pattern to non-pSS. The cold-immune/C2 displayed dramatic activation of classical adaptive immune and depressed metabolism, along with mitochondrial dysfunction and cGAS-STING-NFkB signaling overactivity. “Hot-immune/C3” had an innate immune inclination and predominantly active metabolism processes, especially for oxidative phosphorylation (OXPHOS). Among the three subtypes, C2 had a higher lymphoma risk with the highest immune cell and endothelial cell infiltration in SGs. Based on the distinct features of each group, we matched them with approved or exploratory treatment options the patient most likely would benefit from, including conventional medicine, JAK inhibitors, and biologics.
Conclusion: Overall, our findings provide a comprehensive and in-depth molecular landscape of SGs in pSS, shedding light on the disease heterogeneity and promoting the development of precise clinical intervention strategies for pSS patients.
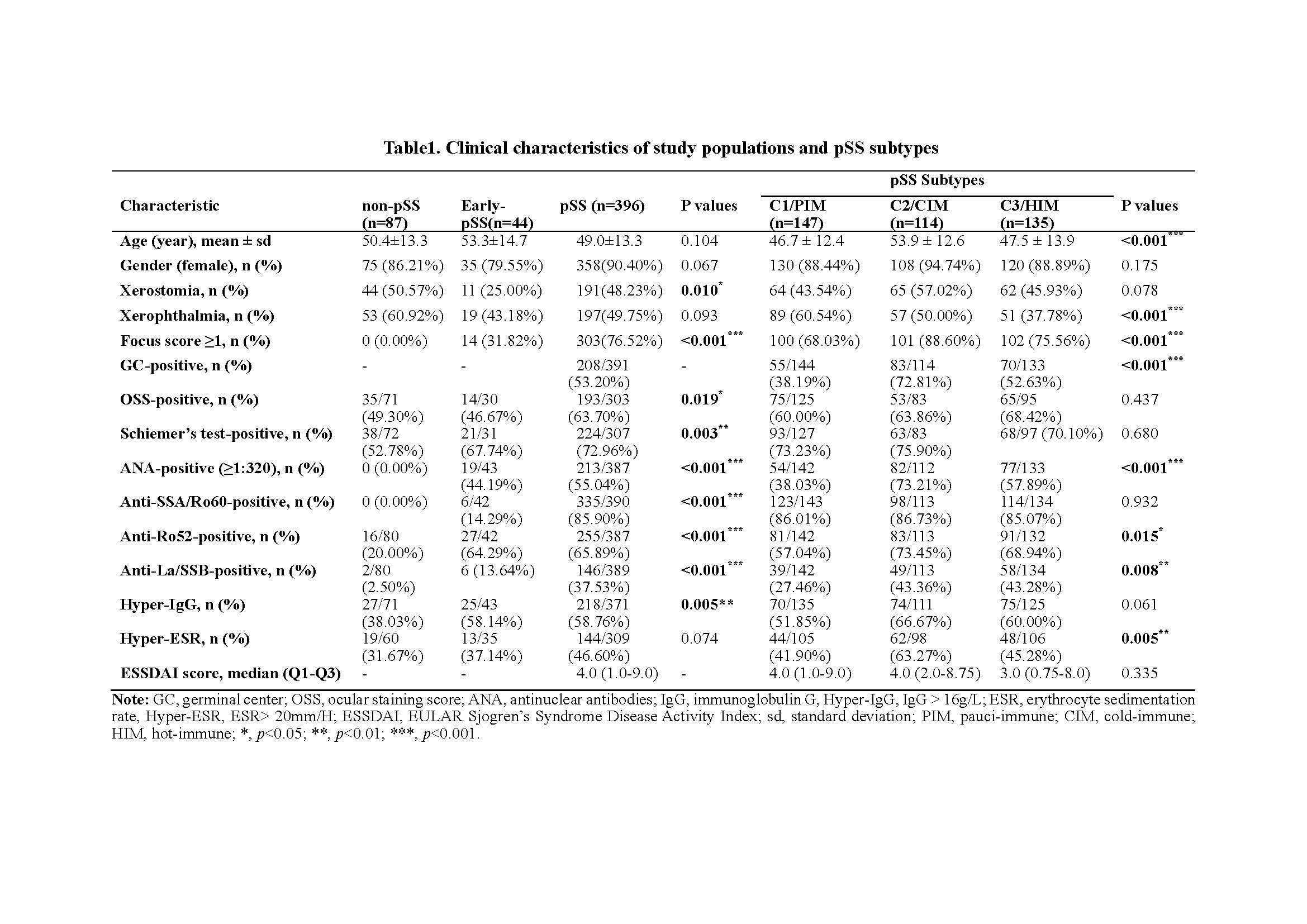
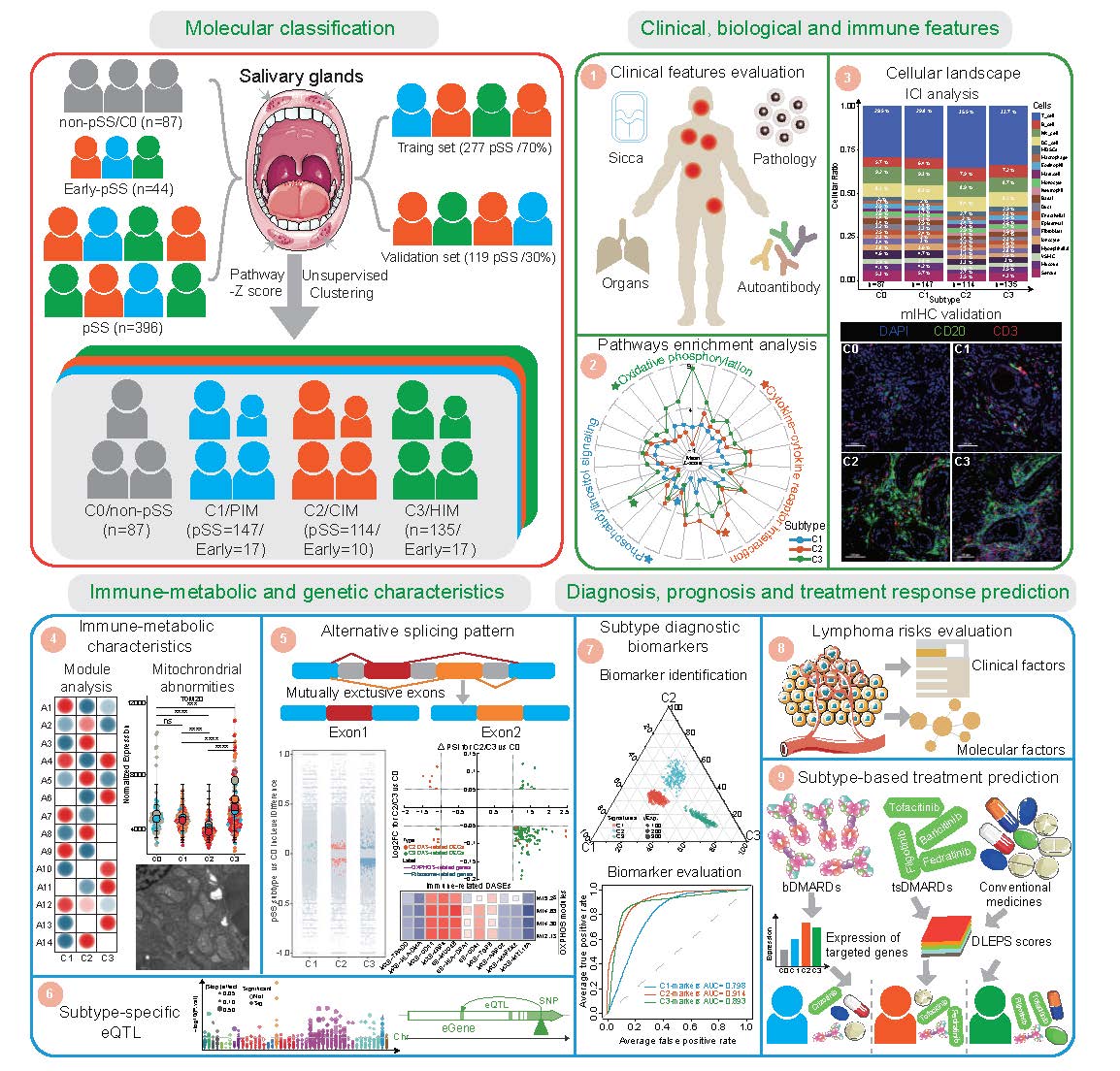
We recruited 527 participants based on respective criteria, including 87 non-pSS (C0), 44 early-pSS, and 396 pSS. Using an unsupervised clustering approach on the transcriptomic data of SGs, we identified three distinct molecular subtypes in pSS and early-pSS. We then conducted extensive downstream analysis on their clinical and biological and immune features, immune-metabolic and genetic characteristics, and disease diagnosis, prognosis, and treatment response prediction.
pSS, primary Sjogren’s syndrome; PIM, pauci-immune; CIM, cold-immune; HIM, heat-immune; ICI, immune cell infiltration; mIHC, multiplex immunohistochemistry; eQTL, expression quantitative trait loci; bDMARDs, biologic disease-modifying anti-rheumatic drugs; tsDMARDs, targeted synthesis disease-modifying anti-rheumatic drugs.
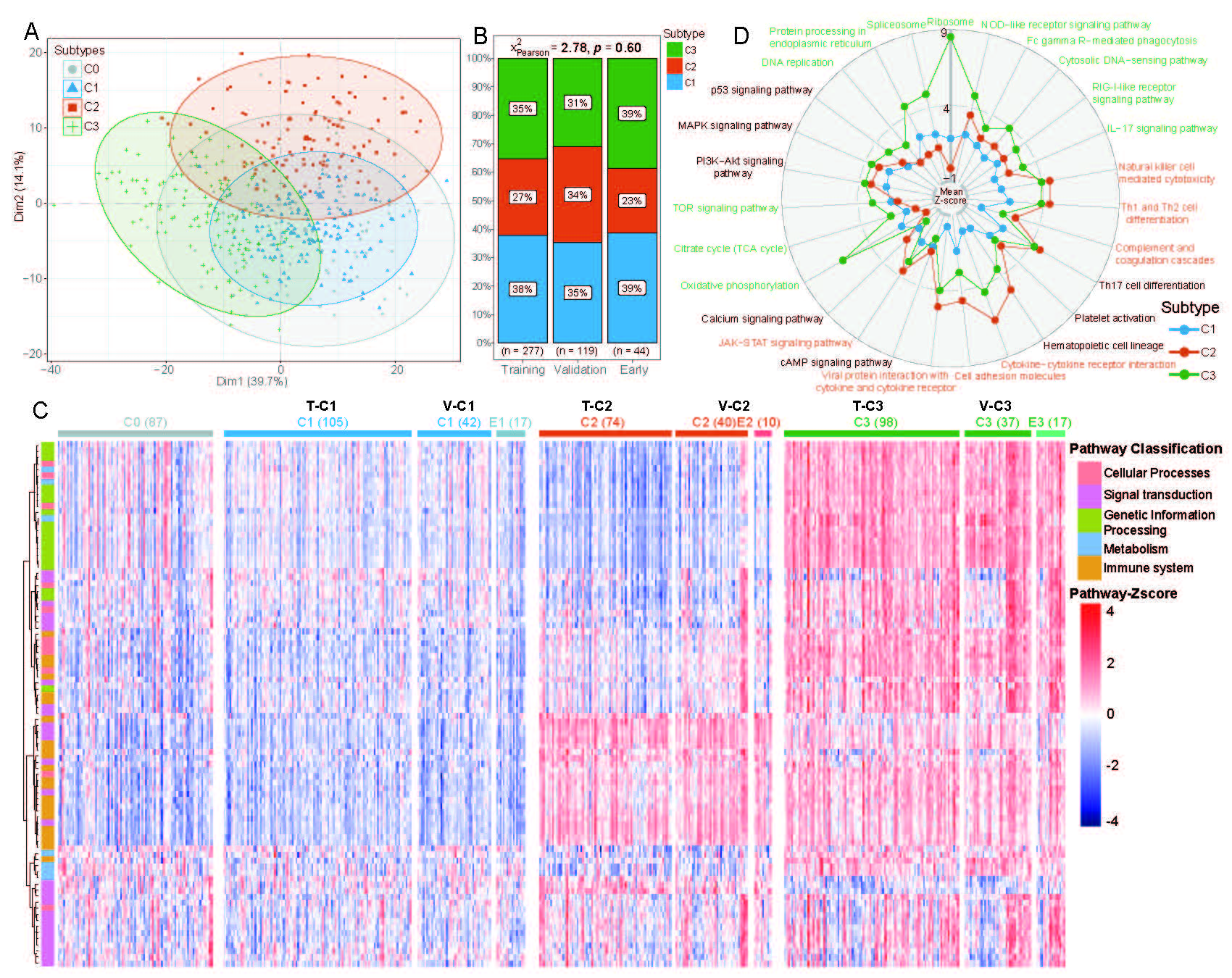
(A) A PCA plot to show 3 groups of pSS patients (C1, C2, C3) by hierarchical pathway-based clustering. Non-pSS cases were overlaid using the same algorithm (C0, grey dots).
(B) The scale map showing the distribution of 3 pSS subgroups in the Training set, Validation set and early-pSS cohorts without significant difference (Chi-Square test, p =0.60).
(C) A heatmap generated from transcriptomic information via hierarchical pathway-based clustering based on z-score levels, including pSS Training set: 277/70%, pSS Validation set: 119/30%, 44 early-pSS patients, and 87 non-pSS cohorts, illustrating the distribution of five categories of pathways across the ten subtypes (non-pSS/C0 (87), T-C1(105), V-C1(42), E1(17), T-C2(74), V-C2 (40), E2(10), T-C3(98), V-C3(37) and E3(17)). T-C represents the clustering pSS subtypes in the Training set; V-C represents the clustering pSS subtypesthe in the Validation set; E represents the clustering early-pSS subtypes.
(D) Representative significant pathways for each cluster. A radar plot showing the mean pathway z-scores in three pSS subtypes; C1 (blue), C2 (red), and C3 (green).
To cite this abstract in AMA style:
Wang X, Luo J, Ying S, Hong J, Cheng H, Wang P, He Y, Ye W, Zhu X, Zhu C, Yang L, Li Z, Lin S, Chen D, Wu X, Xie Z, Wu J, Xu H. Immune-metabolic Heterogeneity and Clinical Implications in Primary Sjogren’s Syndrome Revealed by Molecular Classification of Salivary Glands [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/immune-metabolic-heterogeneity-and-clinical-implications-in-primary-sjogrens-syndrome-revealed-by-molecular-classification-of-salivary-glands/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-metabolic-heterogeneity-and-clinical-implications-in-primary-sjogrens-syndrome-revealed-by-molecular-classification-of-salivary-glands/