Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Olokizumab (OKZ) is a direct interleukin-6 (IL-6) inhibitor. IL-6 is an attractive target for the treatment of pJIA. The aim of the study was to identify the subpopulation of patients with polyarticular-course juvenile idiopathic arthritis (pJIA) who can be expected to have a better treatment response compared to the whole pJIA population.
Methods: Adolescent patients with active pJIA who had failed methotrexate were included in the Phase 2 open-label multicenter study in Russia and received OKZ in a dose of 64 mg every 4 weeks (q4w) subcutaneously (SC) during the main 24-week part of the study. The following outcomes were analyzed: JIA American College of Rheumatology 30/50/70% responses (JIA ACR 30/50/70 responses), Juvenile Arthritis Disease Activity Score-71 (JADAS-71), adverse events (AEs), the associations between the baseline C-reactive protein (CRP) and IL-6 values and effectiveness outcomes. IL-6 was detected in blood with the non-commercial ELISA assay (lower limit of quantification [LLOQ] =2 pg/ml). The significance of change from baseline was tested using the Wilcoxon signed-rank test, and the difference between groups was tested using Fisher exact test.
Results: A total of 16 patients – 9 (56.3%) girls and 7 (43.8%) boys – were enrolled; the median (25%; 75%) age at inclusion was 14.0 (13.0; 16.5) years, at pcJIA onset 11.8 (5.4; 13.7) years, and pcJIA duration was 3.4 (0.4; 8.9). Baseline IL-6 level was >LLOQ in 8 patients, median 87.9 (8.0; 106.9) pg/ml; and in 7 patients was ≤LLOQ. Baseline CRP was >10 mg/l in 8 patients and ≤10 mg/l in 7 patients. By 24 weeks of treatment, median (25%; 75%) JADAS-71 significantly decreased from 20.8 (18.4; 32.6) at baseline to 9.5 (2.65; 20.8) points, p< 0.001. JIA ACR 30/50/70 response was achieved in 80%, 73.3%, 46.7% of patients, respectively. Both JADAS-71 and JIA ACR 30/50/70 outcomes had an insignificant trend toward better results in patients with higher baseline IL-6, with a significant difference for JIA ACR70 (Table 1, Figure 1). In 5 (33.3%) patients inactive disease status was achieved. AEs were reported in 12 (75%) patients, mostly mild to moderate ordinary infections (37.5%), which resolved without complications. No Grade 3-4 AEs, serious AEs,or deaths were reported. One patient discontinued treatment at Week 12 due to de novo psoriasis.
Conclusion: OKZ is a good therapeutic option for the pcJIA that leads to improvement in disease activity and has acceptable safety. It seems that OKZ response may be more successful in patients with higher baseline IL-6 levels.
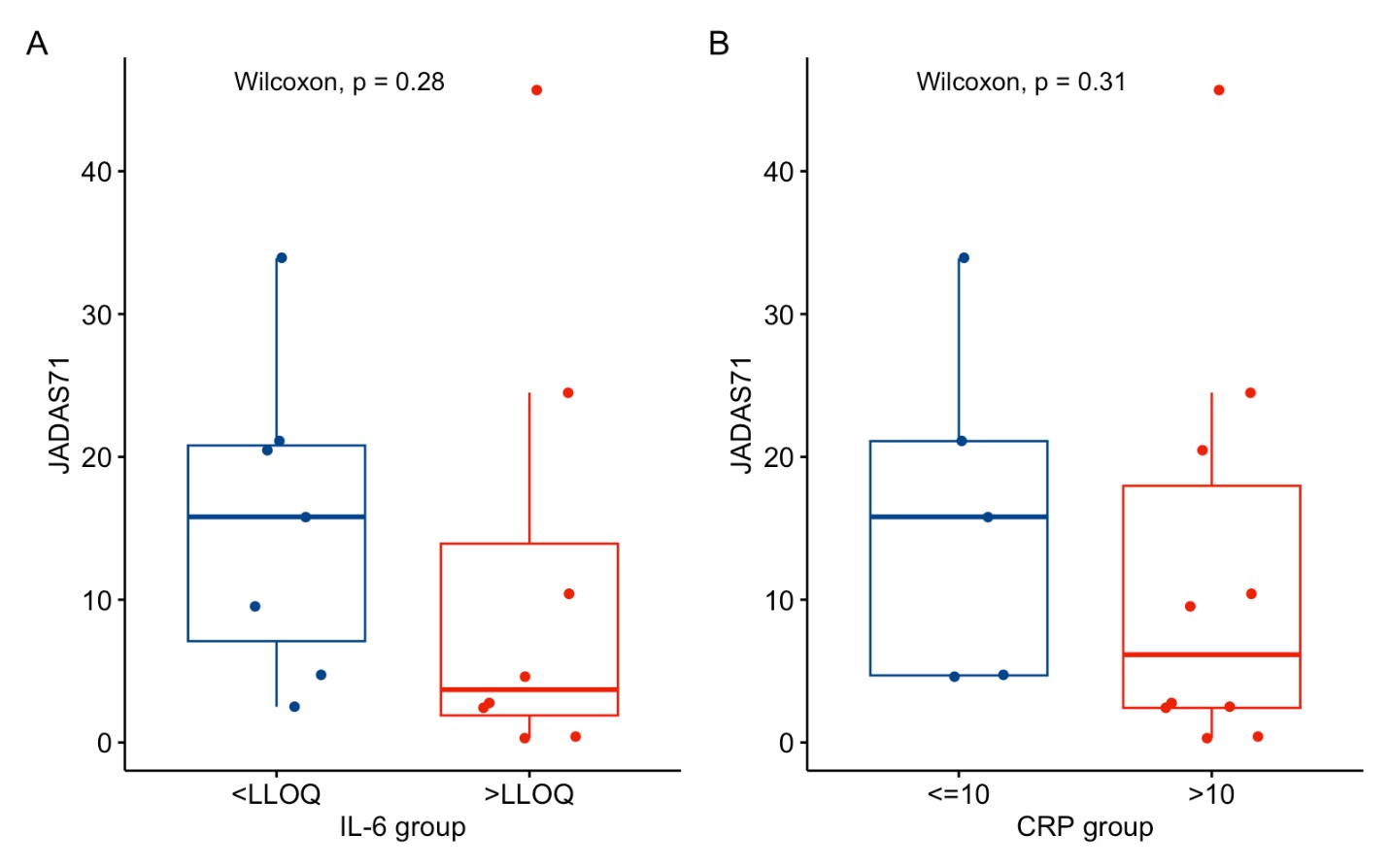 Table 1. Baseline IL-6 and CRP levels vs JIA ACR 30/50/70 responses at Week 24
Table 1. Baseline IL-6 and CRP levels vs JIA ACR 30/50/70 responses at Week 24
.jpg) Figure 1. Baseline IL-6 and CRP levels vs JADAS-71 at Week 24
Figure 1. Baseline IL-6 and CRP levels vs JADAS-71 at Week 24
To cite this abstract in AMA style:
Alexeeva E, Dvoryakovskaya T, Zholobova E, Krekhova E, Matkava V, Raupov R, Bukhanova D, Egorova A, Grishin S, Samsonov M, Kostik M, Nikishina I. IL-6 as a Predictor of Response to Olokizumab in Polyarticular-Course Juvenile Idiopathic Arthritis: Results of the Phase 2 Clinical Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/il-6-as-a-predictor-of-response-to-olokizumab-in-polyarticular-course-juvenile-idiopathic-arthritis-results-of-the-phase-2-clinical-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-6-as-a-predictor-of-response-to-olokizumab-in-polyarticular-course-juvenile-idiopathic-arthritis-results-of-the-phase-2-clinical-trial/
