Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patient-reported outcome measures (PROMs) are essential tools for prioritizing patient-centric care and enhancing health system-performance measurement in rheumatology. Effective use of PROMs collected in clinical care hinges on selecting appropriate measures. The expansion of available PROMs applicable for use in rheumatologic conditions complicates this selection process. Additionally, their use at the point of care remains limited due to barriers in clinical workflows. Engaging patients in the selection process can mitigate these barriers.
The aim of this study was to examine patient preferences and prioritize PROMs for routine collection in RA care as part of a broader health-system quality improvement program.
Methods: We used a modified Delphi consensus approach with a panel of volunteer RA patients. Candidate PROMs were identified through an environmental scan focused on measures relevant to individuals living with RA, including the Patient-Reported Outcomes Measurement Information System (PROMIS). Candidate PROMs met the following criteria: 1) clinical and/or research evidence supporting their use in RA, 2) valid psychometric properties, 3) available at no cost, and 4) feasible to complete in routine care.
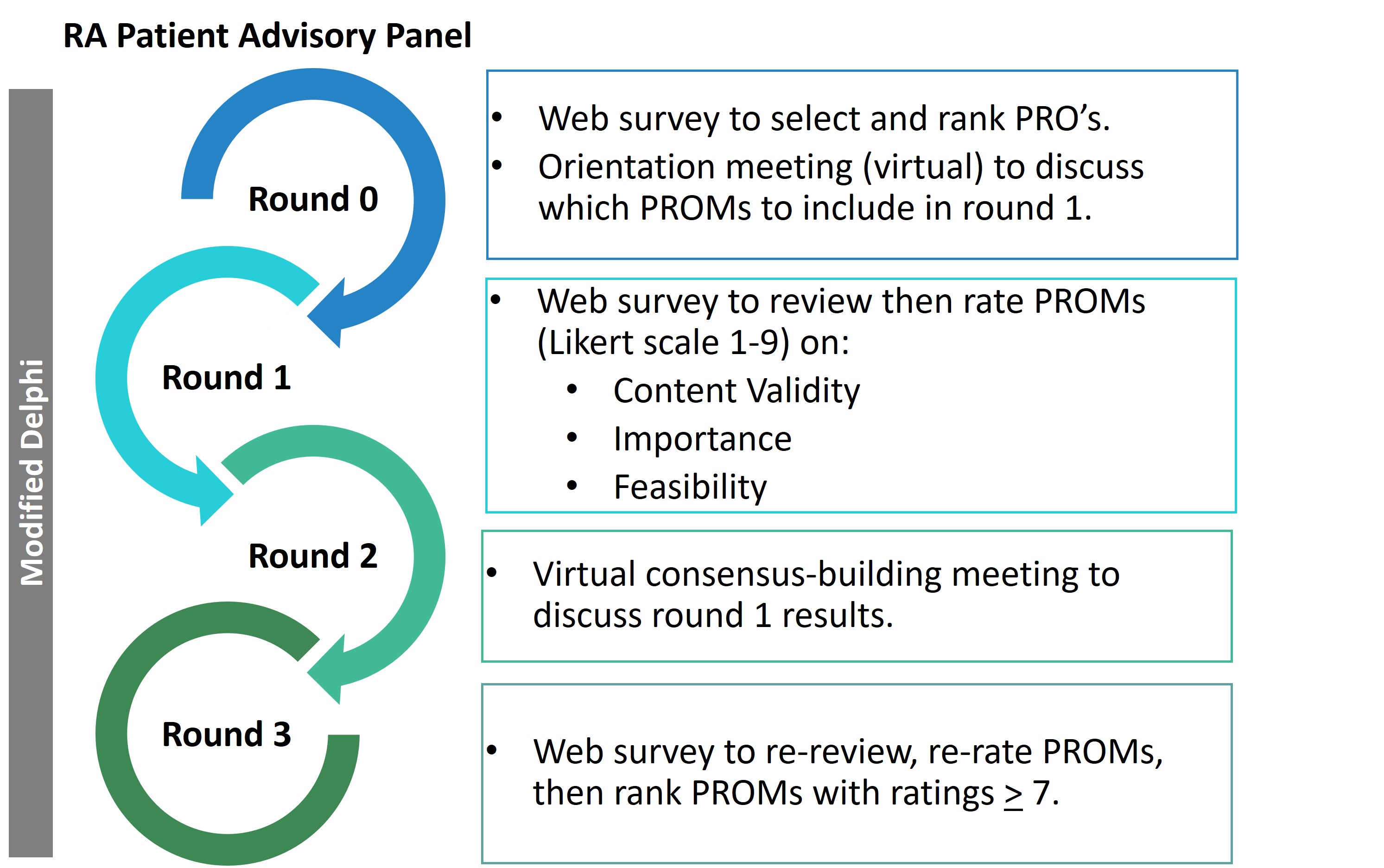
As members of the investigative team, two RA patient co-leads contributed to the study preparatory work and co-facilitation. Additional RA patient volunteers were recruited from tertiary rheumatology care centers and patient-advocacy social media platforms. The Modified Delphi process (Figure 1) began with a preliminary round (Round 0) to familiarize patients with the methodology and terms, and to prioritize health domains/outcomes for review. This was followed by three rounds where participants independently reviewed and rated candidate PROMs on a Likert scale of 1-9 for content validity, importance, and feasibility, with a virtual consensus-building discussion in round 2. Consensus was achieved if PROMs received a median rating of 7 or higher in all criteria and were highly ranked (within the top 5) by participants in the final round. Results were analyzed using descriptive statistics and the overall rank was assigned to PROMs in descending order based on the rank-weighted sum.
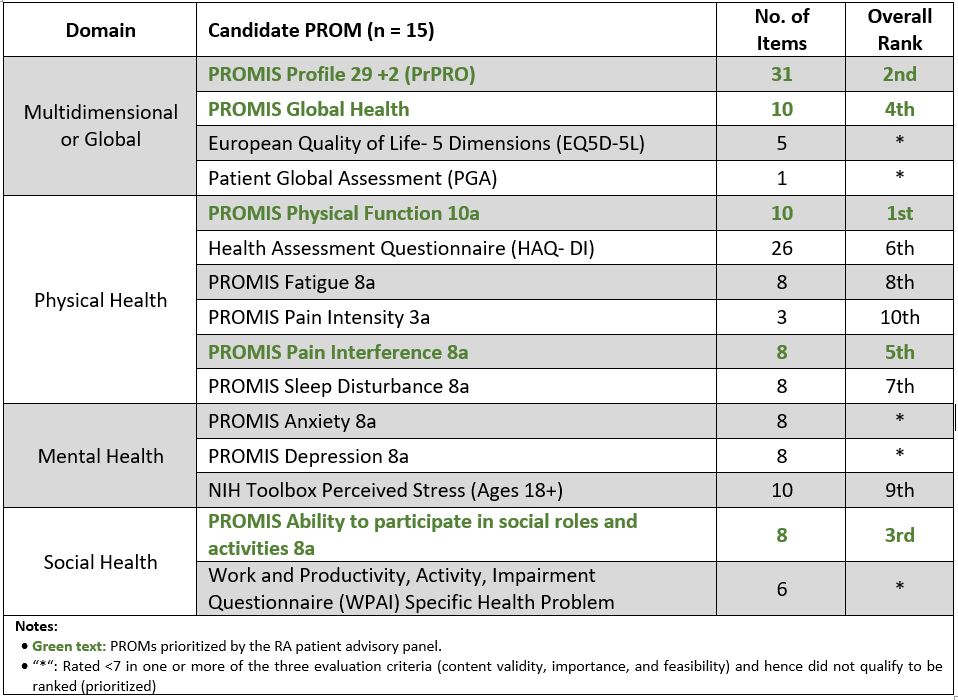
Results: Fifteen RA patients, including the co-leads, took part in the Delphi rounds. Most were female (67%), 45 years old or younger (53%) with a disease duration of < 10 years (67%). From an initial set of 15 candidate PROMS, 10 were rated highly (≥ 7) across all three criteria in Round 3 (Table 1). PROMs ranked within the top 5 included PROMIS Physical Function, PROMIS Profile, PROMIS Ability to Participate in Social Roles and Activities, PROMIS Global Health, and PROMIS Pain Interference respectively.
Conclusion: Patient engagement is critical to ensure that outcomes addressed in clinical practice are aligned with patients’ preferences and priorities. PROMs prioritized by this RA patient advisory panel spanned multiple health domains. Our next steps will include consultations with healthcare providers and health system leaders before finalizing on a set of PROMs for implementation in routine practice.
To cite this abstract in AMA style:
Githumbi R, Katz S, Kania-Richmond A, Giroux K, Wallace Y, Barnabe C, Hazlewood G, Jones C, Steiman A, Ambasta A, Lacaille D, Yacyshyn E, Widdifield J, Gakhal N, Williamson T, Barber C. Identifying Patient Priorities for Patient-Reported Outcome Measures in Rheumatoid Arthritis: A Modified Delphi Consensus Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/identifying-patient-priorities-for-patient-reported-outcome-measures-in-rheumatoid-arthritis-a-modified-delphi-consensus-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-patient-priorities-for-patient-reported-outcome-measures-in-rheumatoid-arthritis-a-modified-delphi-consensus-study/