Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitor (ICI) associated inflammatory arthritis (ICI-IA) has been suggested to occur in approximately 3-6% of ICI-treated patients with cancer1 but most studies of ICI-IA are hampered by small numbers conducted at single centers. In this study, we aimed to identify cases of ICI-IA using administrative claims data to facilitate larger studies.
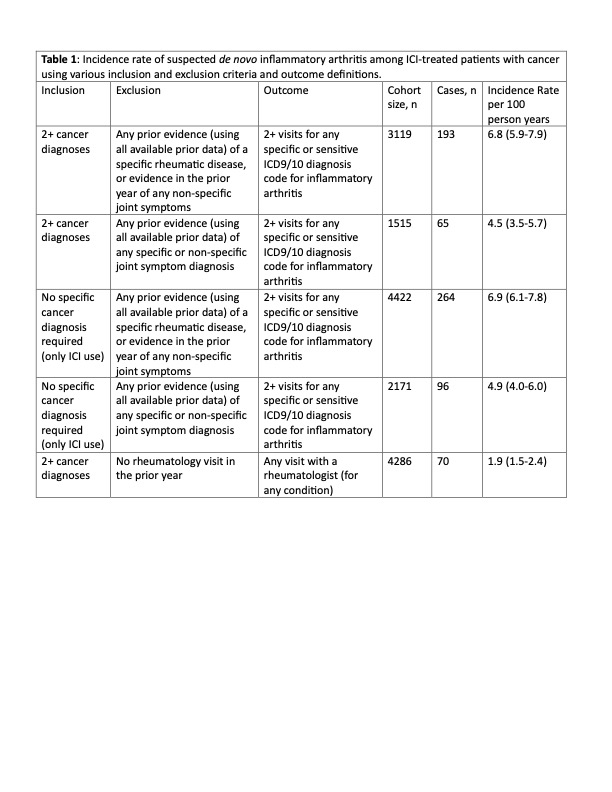
Methods: We used the Medicare 5% random sample from 2015-2019 to identify patients using ICIs. Cancer patients were identified by having at least 2 diagnosis codes from an oncologist for lung cancer, urothelial cancer or melanoma using ICD-9/10-CM codes. ICI-IA was defined as having two Medicare claims at least 30 days apart with combinations of ICD-9/10-CM diagnosis codes that favored specificity. Results were stratified by whether patients had i.) inflammatory arthritis after the date of ICI initiation and ii.) no diagnosis of a rheumatic disease or musculoskeletal complaint in the one year prior to the date of ICI initiation.
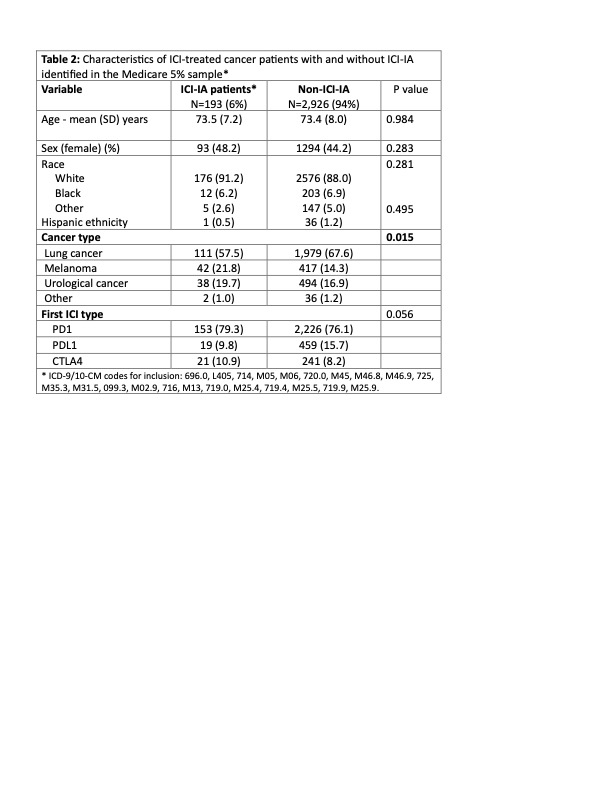
Results: We identified individuals with cancer initiating ICI 2016-2019 with a >= 12-month baseline. The incidence of ICI-IA varied modestly depending on the definition used and was generally in the range of 4-7 per 100 patient years (Table 1). The incidence rates were similar when all cancer were included. Patients with ICI-IA had mean (SD) age 73.5 (7.2) years, 48% women, 91% white (Table 2). More than half of patients (57.5%) had lung cancer, 21.8% had melanoma, and 19.7% urothelial cancer. The median (IQR) time from ICI initiation to first ICI-IA diagnosis was 147 (58, 320) days. The mean (SD) number of provider visits with an ICI-IA diagnoses was 5.3 (6.0), spanning a median (IQR) of 240 (105,461) days. Of the 193 ICI-IA patients identified using our preferred cohort definition, 14.5% used any conventional disease modifying anti-rheumatic drug (DMARD), and fewer than 5% (total) used any TNFi, IL-6Ri, JAKi, or rituximab; none used abatacept. Only 31 (16.1%) received care from a rheumatologist. Median time from ICI initiation to seeing a rheumatologist was 229 (105, 483) days and these patients had a mean (SD) of 11.7 (11.3) visits with the rheumatologist.
Conclusion: We created a simple claims-based algorithm for the preliminary identification of ICI-IA cases using national Medicare claims that results in an incidence similar to that found using registry data.1 While validation of this algorithm using patient level clinical data linked to administrative claims may be useful to identify suspected cases of irAEs, it is likely that more sophisticated methods (e.g. natural language processing run on unstructured data) or manual medical rec
1 Kostine M et al Ann Rheum Dis. 2018 Mar;77(3):393-398.
To cite this abstract in AMA style:
Bass A, Xie F, Meara A, Jannat-Khah D, Curtis J. Identification of Patients with Immune Checkpoint Inhibitor-Associated Inflammatory Arthritis Using Medicare Claims Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/identification-of-patients-with-immune-checkpoint-inhibitor-associated-inflammatory-arthritis-using-medicare-claims-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-patients-with-immune-checkpoint-inhibitor-associated-inflammatory-arthritis-using-medicare-claims-data/